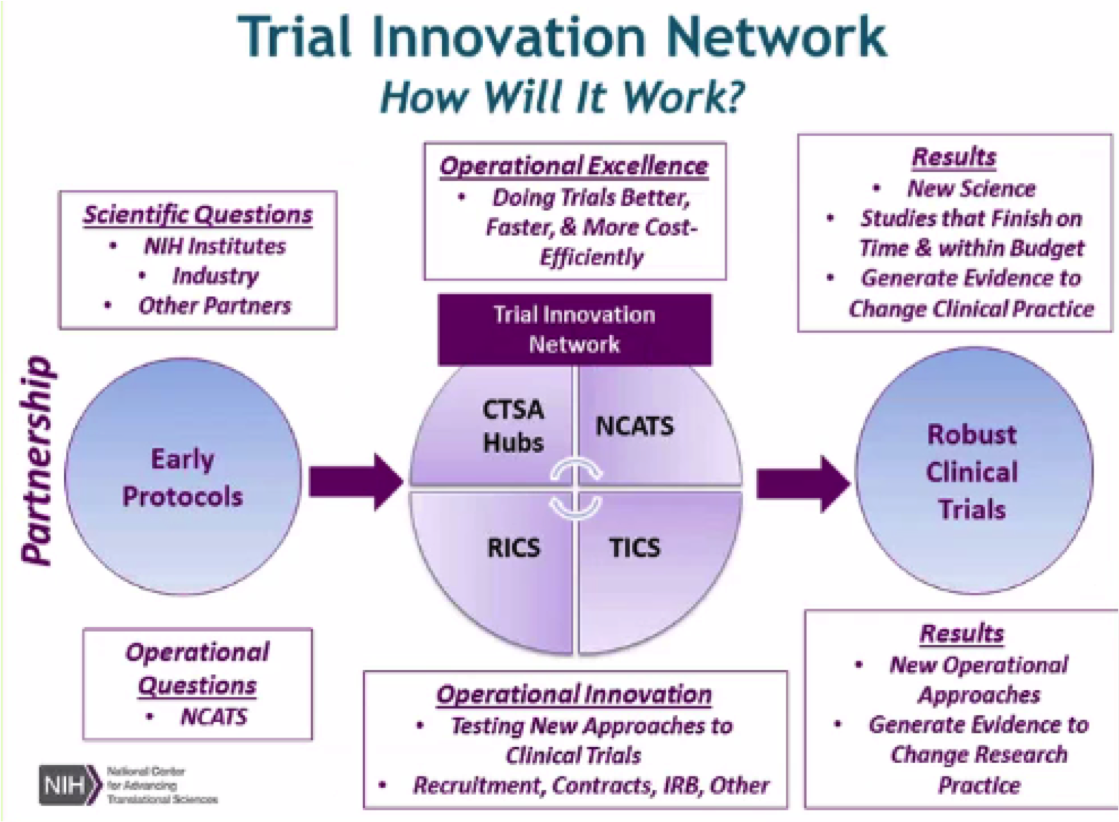
 The NCATS Trial Innovation Network (TIN) seeks to address roadblocks in clinical trials and to accelerate the translation of novel interventions into life-saving therapies. The TIN focuses on innovations in two key areas traditionally associated with creating administrative burdens for multisite studies: trial initiation and patient recruitment. It comprises three key organizational partners:
The NCATS Trial Innovation Network (TIN) seeks to address roadblocks in clinical trials and to accelerate the translation of novel interventions into life-saving therapies. The TIN focuses on innovations in two key areas traditionally associated with creating administrative burdens for multisite studies: trial initiation and patient recruitment. It comprises three key organizational partners:
-
Trial Innovation Centers (TICs)
-
Recruitment Innovation Center (RIC)
-
CTSA Program Hubs
The CCTS Hub has established a TIN Liaison team, which is responsible for coordinating with the national Trial Innovation Network. The TIN Liaison team helps provide reliable study activation, recruitment, retention, and data standards to most effectively facilitate clinical, translational, and comparative effectiveness multisite research focused on improving health outcomes. The team meets weekly and coordinates activities among other multisite study stakeholders, including the IRB, the Office of Sponsored Programs, CCTS Informatics and the Clinical Trials Administrative Office.
The team also interfaces with clinical and translational investigators through the CCTS Research Commons and helps connect the TIN to the capacities of our SHARe platform and so expand the reach of studies to the special populations in our region, which is diverse in ancestry, gender, geographic residence (rural and urban), socioeconomic status and disease burden.
Dr. Jason Nichols, Senior Associate Vice President for Research at UAB, oversees Multisite Study Support (MSSS) on behalf of the CCTS, serving as administrative director of both SHARe and the TIN Hub liaison team. Building on his experience with industry-sponsored clinical trials, Nichols assists in the development of the industry-based research portfolio at the CCTS Hub. He works closely with CCTS investigators across the Partner Network to develop multisite projects and to expedite contract and human subjects review to enable study activation.
Trial Innovation Network Hub Liaison Team
| ROLE | TEAM MEMBER |
|
| Administrative Director |
Jason J. Nichols, OD MPH PhD Senior Associate Vice President for Research (UAB) |
|
| Medical Director |
Robert P. Kimberly, MD Senior Associate Dean for Research Clinical & Translational Research, CCTS Director and Associate VP for Medicine & Biomedical Research |
|
| Program Co-Directors |
Frannie Horn, JD EdS, Program Director, CCTS / SHARe / TIN Hub Jennifer Croker, PhD, CCTS Senior Administrative Director |
|
| Training, Contracting, Regulatory & Recruitment |
Meredith Fitz-Gerald, MSN Director, CCTS Clinical Research Support Program |



