
 |
| Project 1: Division of Labor in Different Subsets of B Cells |
| Once the newly formed B cell exits the generative sites in fetal liver or adult bone marrow it goes through selection events which may involve interactions with self or external antigens. These selective events can influence the fate of individual B cells with respect to their phenotype and functional characteristics. These B cell receptor-mediated events also influence the characteristic sites of localization in lymphoid organs for follicular (B-2 cells) and marginal zone B cells in the spleen, as well as another set (B-1 cells) in the peritoneal and pleural cavities. |
| Compartmentalization of Lymphocytes in the Spleen |
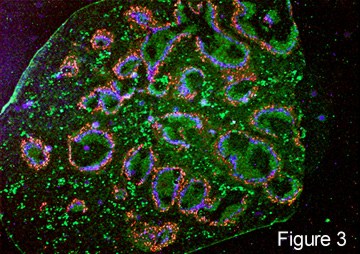
| The spleen is a very important large lymphoid organ consisting of the so-called red pulp which contains macrophages and other cellular elements and is richly bathed in blood entering from a network of sinuses. The white pulp is follicular in nature as shown in a cross section of a mouse spleen in figure 3 and does not contain as many red blood cells hence the name white pulp. These follicles are compartmentalized and various sets of cells reside in special areas in these structures. In the higher power view of one of these (Fig. 4) it can be seen that T cells are found around the arterioles and form the periarteriolar lymphocyte sheath (PALS) (reddish-brown). Adjacent to the T cells are B cell follicles in which B-2 cells reside (green). |
 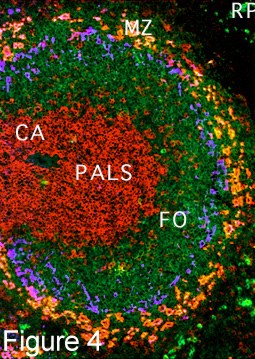  Specialized Macrophages in the Marginal Zone There are then two concentric rings of specialized macrophages Metallophilic macrophages (blue) and marginal sinus macrophages (red). These macrophages are in juxta-position to the open ended marginal sinuses through which blood enters into the parenchyma of the spleen. They are strategically situated therefore to contact and clear foreign particles such as bacteria from the blood the blood. In figure 5, fluorescent bacteria can be seen trapped by these macrophages 30 minutes after their intravenous injection. |
|
Why Are B Cells Different? We are particularly interested in the subset of MZ B cells that reside in this area. We have shown that they have special properties that enable them to respond very rapidly to introduced bacteria by mobilization and differentiation into large clusters of antibody forming plasma cells (Fig. 6). We propose that the compartmentalized distribution of MZ versus FO B cells reflects the different functions associated with each subset. These include the types of antigens recognized by each subset and their roles as antibody producers and antigen presenters.
|
|
In the Future We will determine the relative roles of auto-reactivity, positive and negative selection, in the establishment of fetal and adult B cell repertoires and B cell subsets. By deriving our transgenic mice onto a germ free background we will determine the role of extrinsic antigens on the development of B cell subsets. We will use monoclonal antibodies and molecular techniques to analyze the functional differences between the different B cell subsets in their responses to T-dependent and bacterial T-independent antigens and their roles as antigen presenting cells. Similarly, we will use various cloning strategies to isolate the genes responsible for these functional differences. We will then be in a position to determine if overexpression or dysregulated expression of any of these genes are involved in mechanisms leading to the development of B cell neoplasia within the defined B cell subsets. |
|
Significance of Our Studies These
studies are a continuation
of our long-standing goals over the last 25 years to understand the
cellular
and molecular events during fetal and neonatal B cell differentiation
that
give rise to the adult B cell repertoire. This repertoire includes
long-lived
clones of B cells protective against environmental pathogens. These
studies
will also help us determine whether some of the restricted perinatal B
cell repertoire are
retained in the more highly diversified adult repertoire. We hope to
define the mechanisms that distinguish development of fetal and
neonatal B cells from that of their adult counterparts. Of central
importance is the role that these self-reactive B cells play in the
establishment and maintenance of the normal immune system as well as in
B cell neoplasia, autoimmune diseases, and immunodeficiencies. A
particular interest is to understand the developmental origins and
functions of the B cells and auxiliary cells that participate in the
antibody response to bacterial determinants. |
| References Foote, J.B., and Kearney, J.F. Generation of B Cell memory to the bacterial polysaccharide alpha 1-3 dextran. J Immunol: 183:6359-6368, 2009. Kin, N.W., Crawford, D.M., Liu, J., Behrens, T.W., and Kearney, J.F. DNA Microarray Gene Expression Profile of Marginal Zone versus Follicular B Cells and Idiotype Positive Marginal Zone B Cells before and after Immunization with Streptococcus pneumoniae. J. Immunol: 180:6663-74, 2008. Martin, F., Oliver, A.M., and Kearney, J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity: 14:617-629, 2001. Martin, F. and Kearney, J.F. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity: 12(1):39-49, 2000. You, Y., Myers, R.C., Freeberg, L., Foote, J., Kearney, J.F., Justement, L.B., Carter, R.H. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J Immunol: 2011 Feb 15;186(4):2172-81. Epub 2011 Jan 21. |