Policies aimed at increasing the transparency and reproducibility of federally funded human subjects research continue to roll out, potentially catching investigators by surprise. New requirements announced by the National Institutes of Health (NIH) and the International Committee of Medical Journal Editors (ICMJE) affect multiple stages of the clinical trial lifecycle, from grant writing and implementation to publishing results.
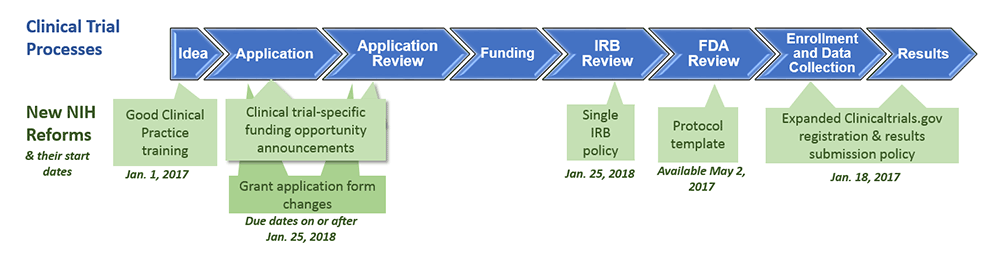
Our May 2018 CCTS Monthly Forum provided a roadmap for navigating the changing clinical trial landscape. In case you missed it, below we reprise what’s changed, what’s ahead, and where to find help when you need it.
Defining a Clinical Trial
Correctly identifying whether a study is considered a clinical trial by NIH will help you select the right NIH funding opportunity for your research, write a stronger grant (especially the research strategy and human subjects sections), and comply with ClinicalTrials.gov registration and reporting requirements once your study is funded and underway.
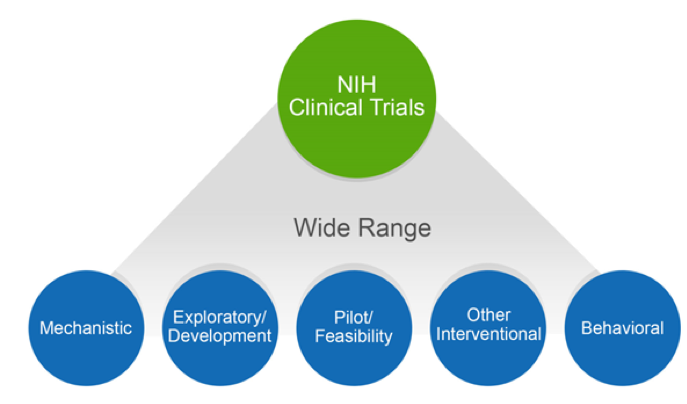
Many types of studies qualify as a clinical trial, including mechanistic, exploratory/development, pilot/feasibility, interventional, and behavioral.
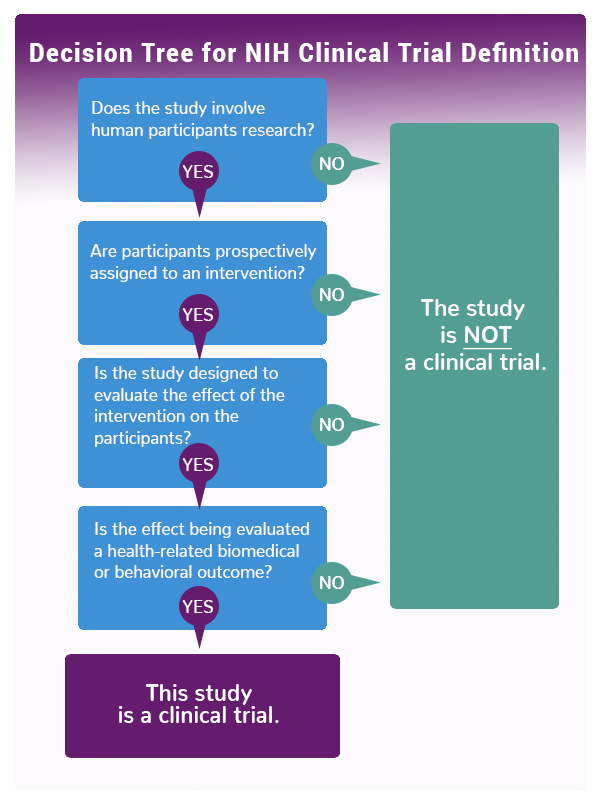
 Answering four questions will help investigators understand how NIH will define their research:
Answering four questions will help investigators understand how NIH will define their research:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answers to the four questions is yes, the study meets the NIH definition of a clinical trial, even if studying healthy participants, lacking a comparison group, or only designed to assess pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug.
Studies intended solely to refine measures or that involve secondary research with biological specimens or health information are not considered clinical trials.
NIH Dissemination Plan
 As of January 18, 2017, all applicants seeking NIH funding must submit a plan for the dissemination of NIH-funded clinical trial information addressing how the expectations of the policy will be met. The plan can be a brief statement explaining whether the applicant intends to register and submit results information to ClinicalTrials.gov.
As of January 18, 2017, all applicants seeking NIH funding must submit a plan for the dissemination of NIH-funded clinical trial information addressing how the expectations of the policy will be met. The plan can be a brief statement explaining whether the applicant intends to register and submit results information to ClinicalTrials.gov.
Visit the UAB Office of Science Policy ClinicalTrials.gov registration page to see an example of a dissemination plan statement.
NIH FORMS-E
As of January 25, 2018, NIH applicants must use FORMS-E, which replaced the old FORMS-D. The new version of the form supports the new definition of a clinical trial; enhances the precision of the information NIH seeks to collect, track, and report; improves dissemination, transparency, and accountability; encourages advances in the design, conduct, and oversight of clinical trials; and aligns with ClinicalTrials.gov fields to better support future data exchanges.
The form consolidates data fields from many different forms into one, the PHS Human Subjects and Clinical Trials Information. Up to 12 attachments may be required by FORMS-E, which is a non-trivial change that CCTS encourages investigators to familiarize themselves with well before a grant deadline.
ICMJE Data Sharing Statement
As of July 2018, data sharing statements are required as a component of all manuscripts submitted to ICMJE journals. More than 1,000 journals follow ICMJE recommendations and requirements.
For clinical trials that start enrolling participants on or after January 1, 2019, as a condition of publication, ICMJE will require investigators to submit a data sharing plan as part of the ClinicalTrials.gov registration process. Data sharing statements are entered in ClinicalTrials.gov’s IPD Sharing Statement module.
Learn More
To access the May Forum slide deck or video, visit our CCTS Monthly Forum page. For questions regarding NIH or ICMJE requirements and/or to request a ClinicalTrials.gov consult, contact the CCTS Clinical Research Support Program (CRSP) or email our CCTS ClinicalTrials.gov expert