Clinical Trials Requirements
NIH launched a multi-faceted effort in 2016 to enhance its stewardship over clinical trials. The goal of this effort is to encourage advances in the design, conduct, and oversight of clinical trials while elevating the entire biomedical research enterprise to a new level of transparency and accountability.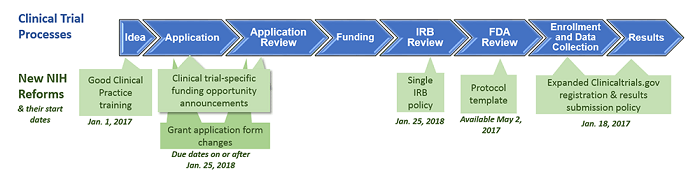 Text version
Text version
Definition of Clinical Trials
The NIH definition of a clinical trial was revised in 2014 in anticipation of these stewardship reforms to ensure a clear and responsive definition of a clinical trial. NIH offers these resources to clarify the definition: case studies, FAQs, decision tree, and related guide notice.
Good Clinical Practice Training
NIH expects all NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials to be trained in Good Clinical Practice (GCP), retain documentation of their training and refresh their training at least every 3 years in order to stay up to date with regulations, standards, and guidelines. NIH policy on GCP training.
New Human Subjects and Clinical Trial Information Form
The new form (FORMS-E) Application Package is used for all submission due dates on or after January 25, 2018. The package includes a new PHS Human Subjects and Clinical Trials Information form that consolidates human subjects, inclusion enrollment, and clinical trial information previously collected across multiple agency forms. The form collects information on human subjects and clinical trials at the study level. General application guide for NIH FOAs.
Single IRB Policy for Multi-site Research
This policy applies to the domestic sites of NIH-funded multi-site studies where each site will conduct the same protocol involving non-exempt human subjects research. It enhances and streamlines the IRB review process for multi-site research so that research can proceed as quickly as possible without compromising ethical principles and protections for human research participants. Policy implementation, expectations and responsibilities.
Registering & Reporting NIH-funded Clinical Trials in ClinicalTrials.gov
All NIH-funded clinical trials are expected to register and submit results information to Clinicaltrials.gov, as per the "NIH Policy on Dissemination of NIH-Funded Clinical Trial Information" for competing applications and contract proposals submitted on or after 1/18/2017. NIH Clinicaltrials.gov registration and reporting policy relates to the federal regulations and decision tree guide for specific actions and checkpoints.
Federal Policy for the Protection of Human Subjects - Revised Common Rule
The U.S. Department of Health and Human Services and fifteen other Federal Departments and Agencies have issued final revisions to the Federal Policy for the Protection of Human Subjects (the Common Rule). The Final Rule was published in the Federal Register on January 19, 2017. It implements new steps to better protect human subjects involved in research, while facilitating valuable research and reducing burden, delay, and ambiguity for investigators. The effective date of the revised Common Rule is January 21, 2019. Revised Common Rule.
Inclusion Across the Lifespan - Policy Implementation
This policy ensures that individuals are included in clinical research in a manner appropriate to the scientific question under study so that the knowledge gained from NIH-funded research is applicable to all those affected by the researched diseases/conditions. The policy expands the Inclusion of Children as Participants in Clinical Research Policy to include individuals of all ages. NIH polices and procedures.
Next Generation Researchers Initiative
This initiative addresses longstanding challenges faced by researchers trying to embark upon and sustain independent research careers, and to take steps to promote the growth, stability and diversity of the biomedical research workforce. Learn more about recent policy changes, the approach, and impact of this initiative. Early stage and early established investigator policies.
NIH Clinical Trials Toolkits
-
National Institute of Dental and Cranialfacial Research (NIDCR): https://www.nidcr.nih.gov/Research/toolkit/
-
National Center for Complementary and Integrative Health (NCCIH): https://nccih.nih.gov/grants/toolbox
-
National Institute of Aging (NIA): https://www.nia.nih.gov/research/dgcg/clinical-research-study-investigators-toolbox/
-
Office of Science Policy: http://osp.od.nih.gov/office-clinical-research-and-bioethics-policy/clinical-research-policy/clinical-trials
-
National Center for Advancing Translational Sciences: https://ncats.nih.gov/expertise/clinical
-
Office of Research on Women's Health: How to Engage, Recruit, and Retain Women in Clinical Research: https://orwh.od.nih.gov/toolkit/recruitment
Webinar - The Eureka Research Platform: A Resource for Mobilizing Research
This NIH Office of Behavioral and Social Sciences Research (OBSSR) Director’s Webinar, recorded May 14, 2019, features guest presenter Jeffrey Olgin, M.D., Gallo-Chatterjee Distinguished Professor of Medicine and chief of the division of cardiology at the University of California, San Francisco. Dr. Olgin, provides an overview of the Eureka Research Platform, an NIH-funded resource for conducting research using mobile technology. He describes the resource, including its capabilities, provides a description of ongoing studies using the platform, and shares lessons learned and the mechanisms by which the resource can be used for NIH-funded studies. Watch the video on YouTube.
Optimizing the Investment in Medical Devices for Rehabilitation
The National Center for Medical Rehabilitation Research (NCMRR) convened stakeholders to determine best methods to work with investigators developing devices and assistive technologies for the medical rehabilitation market to clear regulatory and payer hurdles for market entry and successful commercialization. The theme of the workshop was to identify success stories of rehabilitation devices that have entered the market for clinical application or for direct use by the individual with disability. The group identified needed resources, infrastructure, and information needed to build on these successes and identified avenues for dissemination of these throughout the research community.
Presentations: Day 1 and Day 2
The Future of Medical Rehabilitation Clinical Trials
The NCMRR and the NIH National Rehabilitation Research Resource to Enhance Clinical Trials (REACT) Center cohosted this workshop, at the NIH campus in Bethesda, Maryland. Led by Dr. Allison Cernich, Former Director, NCMRR, and Dr. Marcas Bamman, the two-day workshop featured presentations and lively discussions by rehabilitation researchers and clinical trialists from around the nation. The workshop aimed to take a closer look at how clinical trials in medical rehabilitation are unique, and how they can be bolstered by best practices, collaborative research efforts, and innovative funding resources.
Presentations: Day 1 and Day 2
Rehabilitation Research at NIH: Moving the Field Forward
Rehabilitation research took center stage at this National Institutes of Health–sponsored conference, hosted on the NIH campus in Bethesda, Maryland. From a memorable keynote performance by teens and young adults with disabilities to moving remarks by NIH Director Francis Collins, the conference generated tremendous enthusiasm and helped bring about a research plan for future initiatives in medical rehabilitation.
Presentations: Day 1 and Day 2
Access the executive summary published in Archives of Physical Medicine and Rehabilitation.