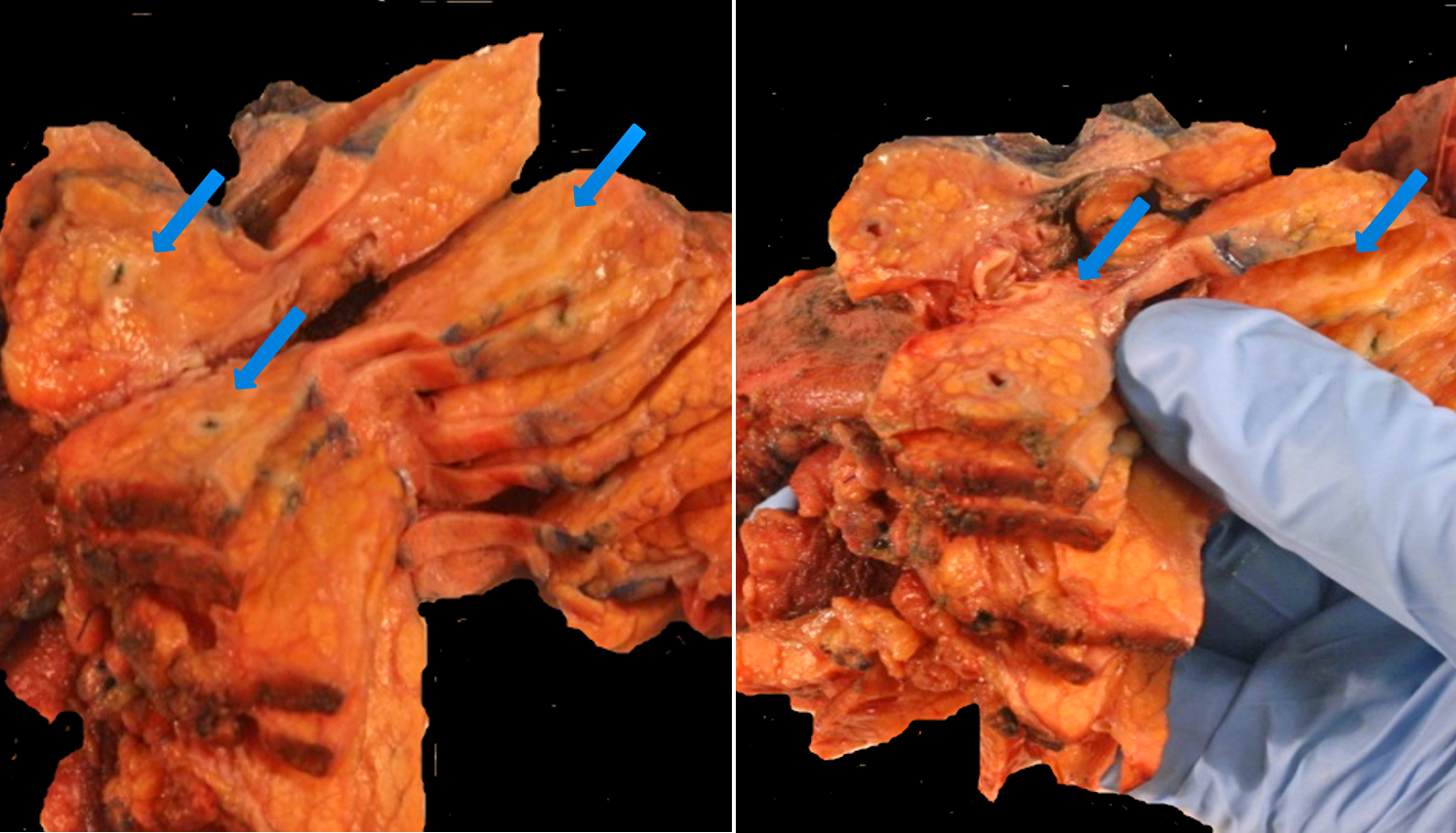
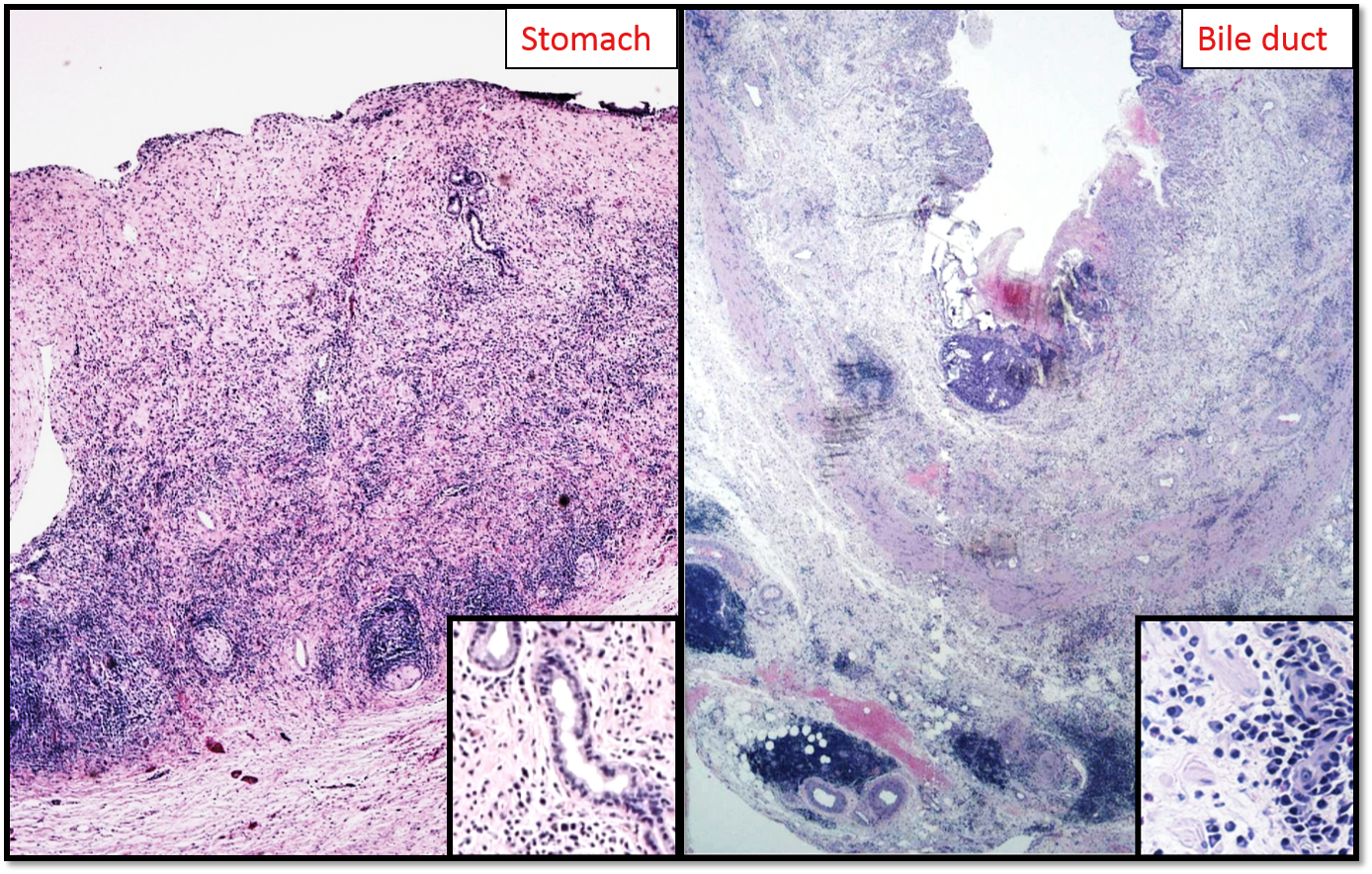
A 65-year-old male presented with abdominal pain and 4-5 pounds weight loss, but no biliary obstruction. His extensive workup revealed gastritis and a mass in the head of the pancreas, 2.7 cm. Outside BX reported to be a tumor mucinous in character. The patient underwent proximal subtotal pancreatectomy with a Whipple procedure.
What is your diagnosis of this case?
- Multiple myeloma
- Autoimmune pancreatitis (AIP), type 2
- Inflammatory bowel disease/CD involving the pancreas
- IgG4-related sclerosing disease (IgG4-RSD)
Answer: D. IgG4-related sclerosing disease (IgG4-RSD)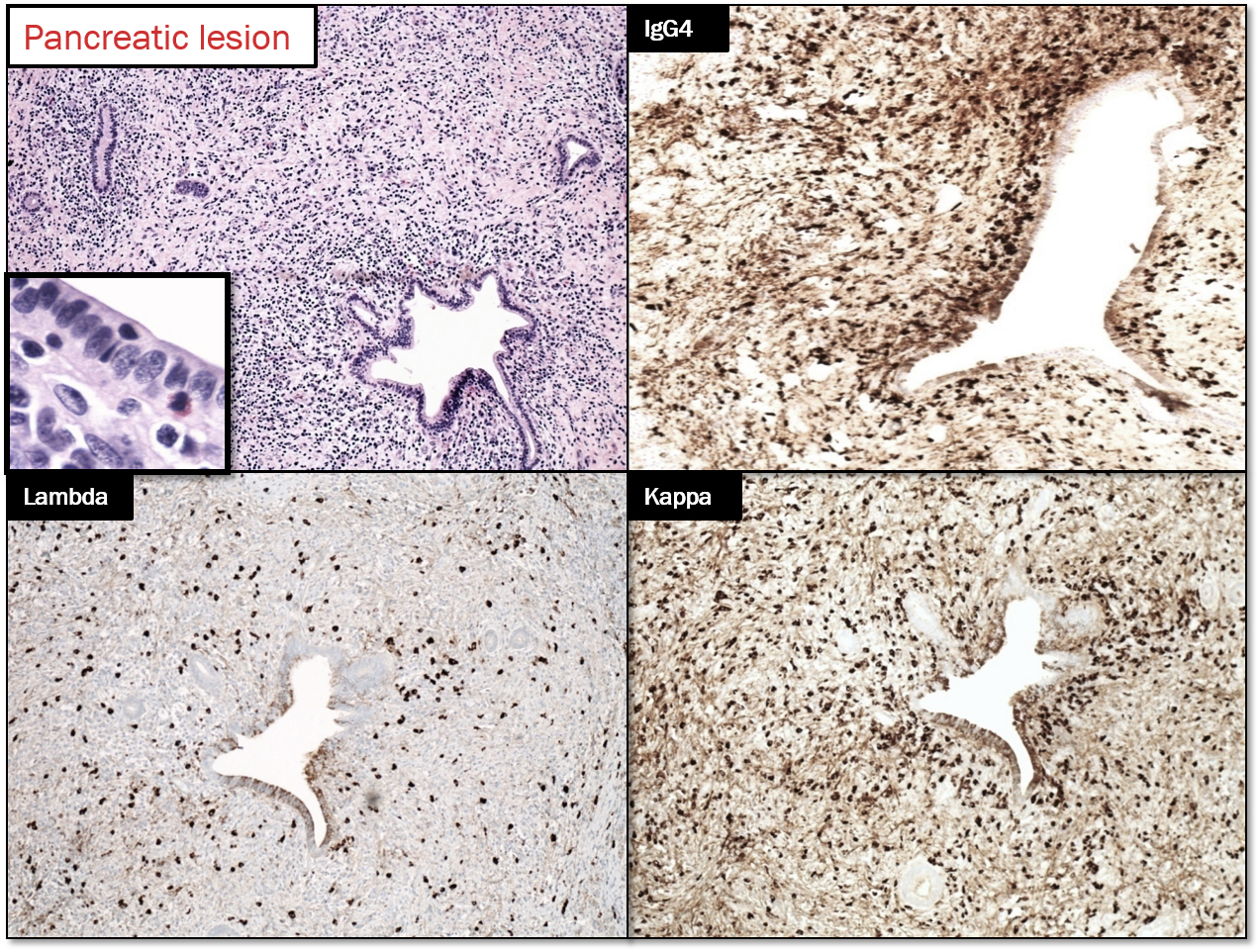
Discussion
IgG4-RSD disease has characteristic but not specific histologic changes, which includes: lymphoplasmacytic inflammation, fibrosis, phlebitis and increased numbers of IgG4-positive plasma cells make up the characteristic mix. [1].
Chronologically, Chari et al described the association of certain subsets of chronic pancreatitis with autoimmune diseases and proposed that these be classified separately as ‘chronic autoimmune pancreatitis’. Shortly after that, Yoshida et al recognized the concept of autoimmune pancreatitis (AIP) by representing a rapid response to steroids in a case of chronic pancreatitis with elevated gammaglobulin levels. Kamisawa et al. described that similar histologic changes i.e., infiltration of tissues with IgG4 positive plasma cells along with storiform fibrosis and obliterative phlebitis occurred in multiple sites e.g., bile ducts (now known as IgG4-associated cholangitis or IAC), salivary glands (chronic sclerosing sialadenitis), kidney (tubulointerstitial nephritis), retroperitoneal fibrosis and concluded that AIP is indeed a multisystem IgG4-related disease (IgG4 RD). [2] Notohara et al termed them “lymphoplasmacytic sclerosing pancreatitis (LPSP)” and“idiopathic duct-centric pancreatitis (IDCP)”.[3] Despande et al chose the terms lobulocentric AIP and duct –centric AIP [4]. The two patterns have come to be called type 1 and type 2 AIP respectively. [5,6]
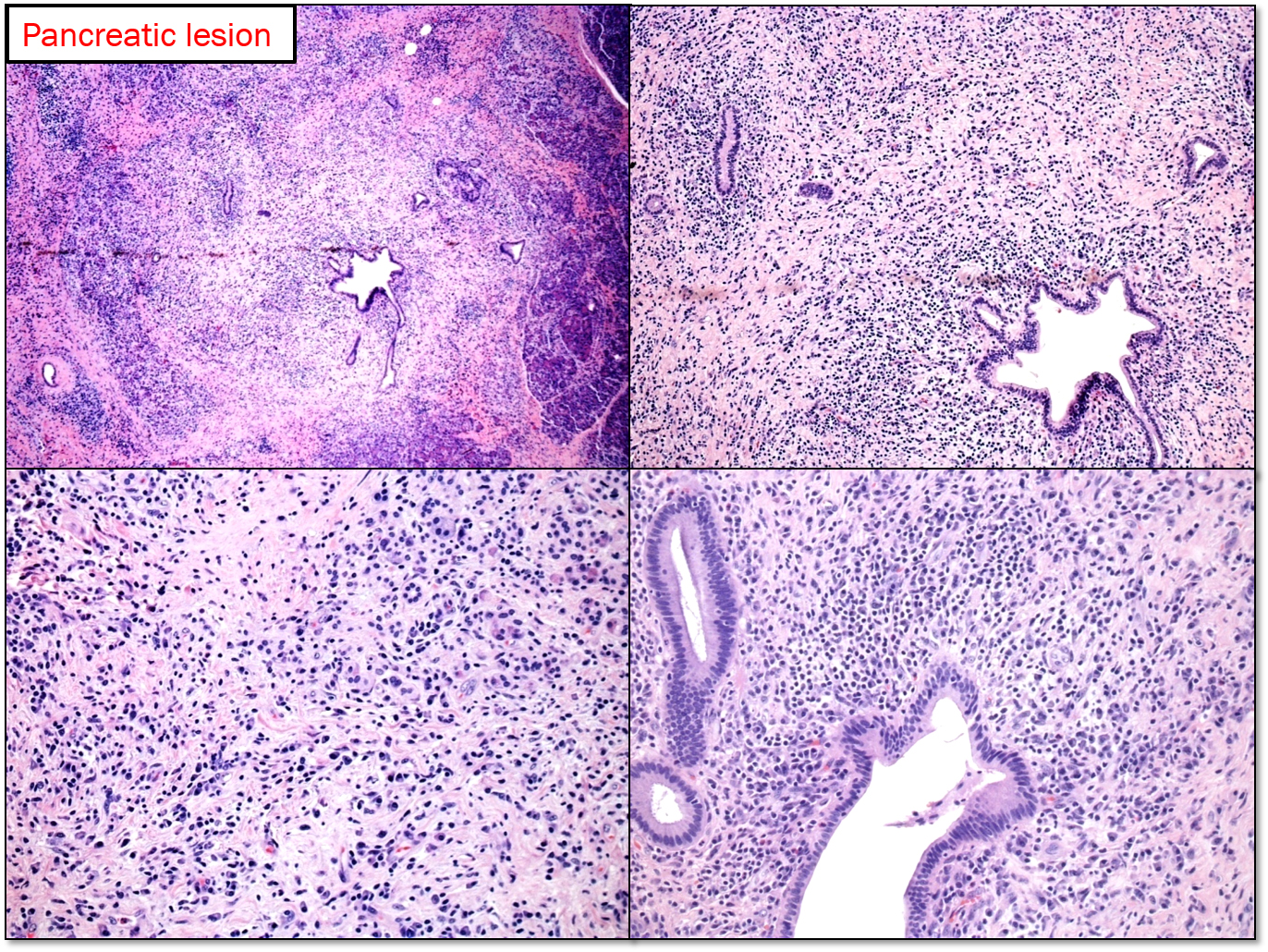
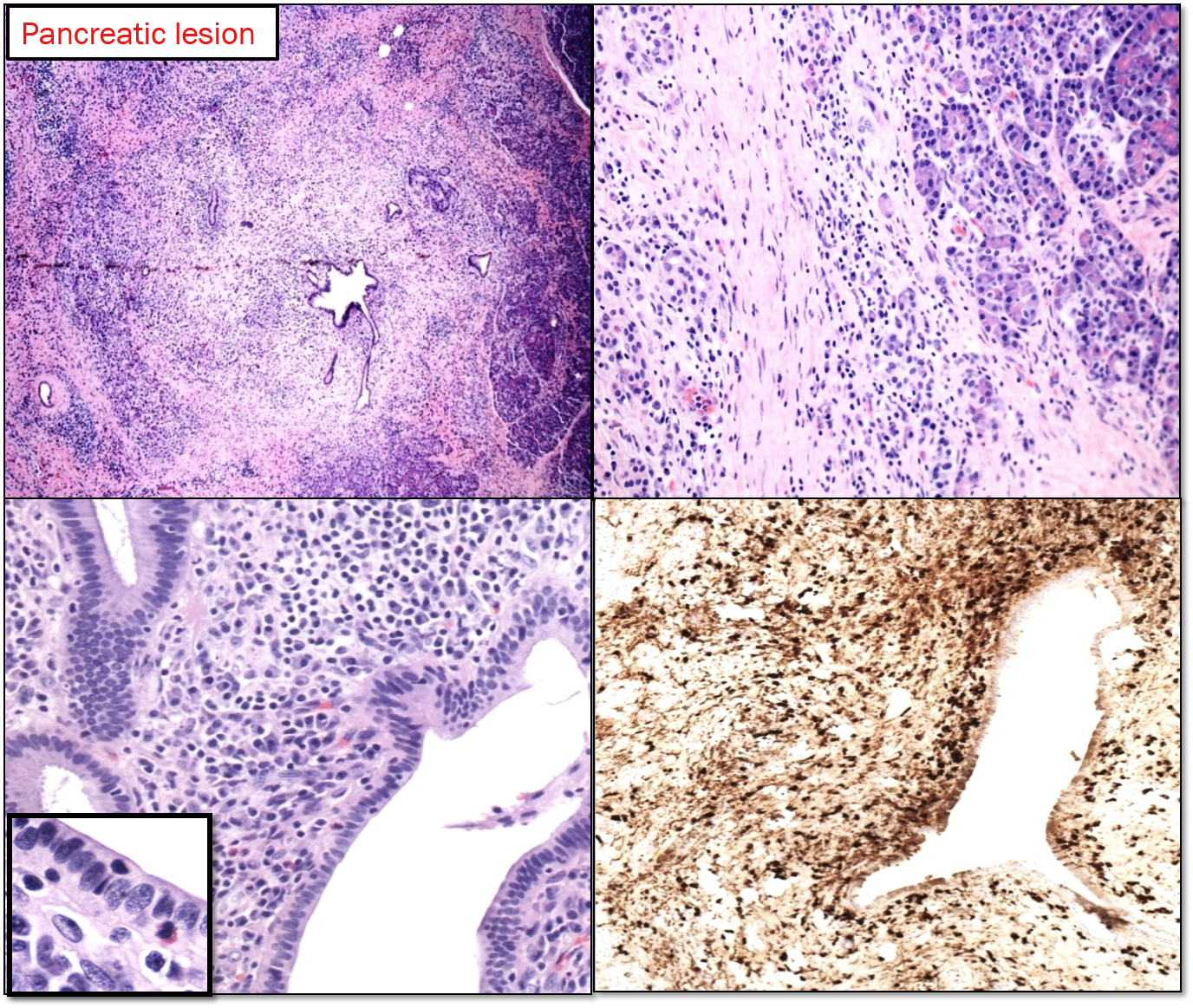
Type 1 AIP: It is the pancreatic manifestation of IgG4-related sclerosing disease. The affected patients are generally males (M: F ratio is 4:1) in their 7th decade. It usually involves the entire pancreas, but can be localized enough to mimic pancreatic tumor. There is usually a cuff of lymphocytes and plasma cells surrounding branches of the pancreatic duct. While the inflammatory cuff can be quite dense, the duct epithelium remains intact. Neutrophils are not seen in or around the epithelium; in fact, the presence of intraepithelial neutrophils favors type 2 AIP over type 1. Fibrosis [storiform pattern, which tends not to occur in other forms of chronic pancreatitis (including type 2 AIP) or in the pancreatitis adjacent to neoplasm, making it a very helpful diagnostic feature, particularly in needle biopsies.] Phlebitis is invariably seen in resected examples of type 1 AIP. The vein lumen is narrowed (sometimes completely obliterated) by a dense infiltrate of lymphocytes and plasma cells. [8]
An IgG4-positive infiltrate alone is not specific for a diagnosis of type 1 AIP (or any particular manifestation of IgG4-RSD), but in the appropriate clinicopathologic setting, positive staining for IgG4 can settle the diagnosis. In the resection specimens, cut-off value of 50 IgG4-positive cells/hpf gave a sensitivity for AIP of 84% and a specificity of 100%. [9]. IgG4+/IgG+ plasma cells = >40%. [10]. Peripancreatic lymph nodes usually show a distinctive IgG4-rich hyperplasia in AIP1, but are not abnormal in AIP2.
It is important to keep in mind that there are non-IgG4-RD cases with increased IgG4+ cells. Inflammatory conditions (including primary sclerosing cholangitis and inflammatory bowel disease), pancreatobiliary carcinomas and lymphoma.
Radiologically, computed tomography (CT) scan shows a diffuse enlargement and effacement of the usual lobular appearance. Endoscopic retrograde cholangiopancreatography shows à diffuse or long segments of irregular narrowing of the main pancreatic duct. Some cases, clinical presentation and radiographic appearance mimics pancreatic carcinoma and leads to pancreatic resection as in the case presented here.
Diagnosing type 1 AIP in small biopsies: The fact that this is a non-neoplastic, steroid-responsive condition means that we are now being asked to give the diagnosis based on needle biopsies. This will help to avoid unnecessary surgical resection. Assuming that the biopsy shows chronic pancreatitis and no evidence of malignancy, one can usually point the diagnosis in favor of or against type 1 AIP. Even small duct branches, if present, will often show a dense periductal lymphoplasmacytic infiltrate. If vein branches are present, phlebitis is a characteristic finding. In practice, neither ducts nor veins are commonly seen in needle biopsy; in that case, the cell-rich storiform fibrosis becomes the critical finding. Immunohistochemistry for IgG4 is invaluable in this setting. Increased numbers of IgG4-positive plasma cells (more than 10/hpf), particularly if they are diffuse, strongly support the diagnosis of type 1 AIP.
Diagnosing type 1 AIP by FNA: Some studies have shown that fine needle aspiration of the pancreas can provide adequate diagnostic material. [11] By recognizing stromal fragments on cytology preparations. Usually the inflammation of type 1 AIP makes the stromal fragments more cellular than in carcinoma.
Type 2 AIP: The patients are younger than those with type 1. Type 2 AIP involves male and female equally. It is confined to the pancreas. Systemic manifestations of IgG4-RSD are absent. Peripancreatic lymph nodes are not abnormal in AIP2 but usually show a distinctive IgG4-rich hyperplasia in AIP1. [12]
While the ducts are cuffed by dense lymphoplasmacytic inflammation, the epithelium contains neutrophils. The neutrophils often form microabscesses. The characteristic histological features of type 1 AIP (storiform fibrosis, phlebitis, lymphoid aggregates in peripancreatic parenchyma) and IgG4-positive cells are either poorly developed/sparse or absent in type 2 AIP.
Summary:
| Type 1 AIP | Type 2 AIP | |
| Age/gender (M:F) | 7th decade/ (4:1) | 5th decade/ (1:1) |
| Extra pancreatic /IgG4 RD | Yes | No |
| Association with IBD | Weak | Strong |
| Lymphoplasmatic /Periductal inflammation | Yes | Yes |
| Neutrophilic epithelial infiltrates | No | Yes/characteristic |
| Storiform fibrosis | Prominent | Less |
| Phlebitis | Yes/characteristic | Rare |
| IgG4 staining | Abundant, >10/hpf | Rare, <10/hpf |
| Response to steroids | ~ 100% | ~ 100% |
References:
[1] Cheuk W, Chan JK. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv Anat Pathol. 2010 Sep;17(5):303-32.
[2] Nagpal SJS, Sharma A, Chari ST. Autoimmune Pancreatitis. Am J Gastroenterol.
2018 Jun 18. doi: 10.1038/s41395-018-0146-0. [Epub ahead of print] Review. PubMed PMID: 29910463.
[3] Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003 Aug;27(8):1119-27.
[4] Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, et al. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006 Dec;30(12):1537-45.
[5] Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010 Jul;39(5):549-54.
[6] Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006 Aug;4(8):1010-6; quiz 934.
[7] Chu KE, Papouchado BG, Lane Z, Bronner MP. The role of Movat pentachrome stain and immunoglobulin G4 immunostaining in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2009 Mar;22(3):351-8.
[8] Bledsoe JR, Della-Torre E, Rovati L, Deshpande V. IgG4-related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018 Jun;126(6):459-476. doi: 10.1111/apm.12845. Review. PubMed PMID: 29924455.
[9] Dhall D, Suriawinata AA, Tang LH, Shia J, Klimstra DS. Use of immunohistochemistry for IgG4 in the distinction of autoimmune pancreatitis from peritumoral pancreatitis. Human Pathology. 2010 May;41(5):643-52.
[10] In J Rhematol 2012; 2012:580814.
[11] Deshpande V, Mino-Kenudson M, Brugge WR, Pitman MB, Fernandez-del Castillo C, Warshaw AL, et al. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: diagnostic criteria and pitfalls. Am J Surg Pathol.2005 Nov;29(11):1464-71.
[12] Deshpande V GR. Subclassification of autoimmune pancreatitis: A histologic classification with clinical significance. Am Surg Pathol. 2011 January 2011;35:526-35.
Case contributed by Sameer Al Diffalha, M.D., Assistant Professor, Laboratory Medicine