Displaying items by tag: department of microbiology
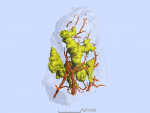
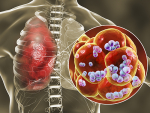
MicroCT of infected human lung tissue, along with histology and immunohistochemistry, was used to construct images of TB granulomas, airways and vasculature.
Tagged under
Intranasal vaccination is needle-free and elicits immunity at the site of infection, the respiratory tract.
Tagged under
This novel virulence trait, which increases severity of S. pneumoniae superinfection, involves pneumococcal surface protein A, now identified as an adhesin.
Tagged under
A comprehensive health-screening program in rural northern KwaZulu-Natal has found a high burden of undiagnosed or poorly controlled non-communicable diseases.
Tagged under
Immunity levels are keeping state case levels manageable for now, but the current vaccination rate will not end COVID and likely will lead to continued outbreaks.
Tagged under
- release
- school of medicine
- uab medicine
- division of infectious diseases
- department of medicine
- hugh kaul personalized medicine institute
- department of pathology
- department of microbiology
- college of arts and sciences
- alabama vaccine research clinic
- school of engineering
- school of education
- graduate school
- school of optometry
- school of dentistry
- school of nursing
- school of public health
- school of health professions
- collat school of business
- coronavirus
The COVID-19 pandemic highlighted the urgent need for enhanced study and discovery in the field of immunology.
Tagged under
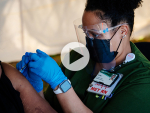
A single intranasal dose provided sterilizing immunity — no detectable COVID-19 virus — in the lungs of vaccinated mice, in contrast to dense infection in lungs of unvaccinated mice.
Tagged under
Knowledge of the specific flagellins that drive the pathogenic immune response in Crohn’s disease is a step toward a potential preventive treatment.
A bilingual speaker of Japanese, Leanna Miku Crafford volunteered with a nonprofit health care clinic, did research in microbiology and led the Japanese Culture Club. She will graduate May 1 with honors magna cum laude with a Bachelor of Science degree in biology.
Tagged under
This transport system may be widespread across many Gram-positive bacteria that contain proteins in the WXG100 superfamily. Tuberculosis kills 1 million people each year.
Tagged under
This study will aid the understanding of, and future research on, inflammatory bowel disease, which afflicts about 1.6 million Americans.
Tagged under
The list was made by a group that aspires to bolster and increase diversity across all scientific fields, promote retention through the “leaky academic pipeline,” and broaden academic and industrial awareness of diversity and inclusion.
Tagged under
- release
- neuroscience
- school of medicine
- school of public health
- college of arts and sciences
- department of chemistry
- department of neurology
- department of medicine
- department of epidemiology
- department of psychology
- department of microbiology
- department of neurobiology
- department of physics
- division of endocrinology diabetes and metabolism
- division of hematology and oncology
- comprehensive neuroscience center
- oneal comprehensive cancer center
Preclinical tests at UAB last year showed potent systemic and mucosal immune responses in mice after a single intranasal dose. The vaccine candidate was developed by Maryland-based Altimmune Inc.
Tagged under
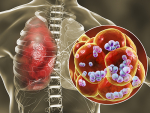
Streptococcus pneumoniae is an opportunistic pathogen that commonly infects young children and the elderly. This atlas will help researchers better understand how to treat these infections.
Tagged under
Tagged under
Maryland-based Altimmune Inc. has submitted an Investigational New Drug application to the U.S. Food and Drug Administration to commence a Phase 1 clinical study of its single-dose intranasal candidate.
Tagged under
Tagged under
High-profile speakers show the “critical role that UAB is playing in the effort to combat this pandemic.”
Tagged under
Layers of tannic acid and another biopolymer delay allograft and autoimmune-mediated rejection in mouse models of Type 1 diabetes.
Tagged under
A monoclonal antibody is being developed by Aridis Pharmaceuticals as an inhaled, self-administered treatment for non-hospitalized patients who are suffering from mild to moderate COVID-19.
Tagged under