University of Alabama at Birmingham Frances Lund, Ph.D. researchers will receive $17.8 million in federal funding to attack a key “knowledge gap” in human immunology — how the B cells and antibody-secreting cells that reside in tissues and organs differ from those found in blood. It is an exploration never undertaken in a systematic way, studying many individual people.
Frances Lund, Ph.D. researchers will receive $17.8 million in federal funding to attack a key “knowledge gap” in human immunology — how the B cells and antibody-secreting cells that reside in tissues and organs differ from those found in blood. It is an exploration never undertaken in a systematic way, studying many individual people.
These unknown differences among the immune cells may alter resistance to infections, and they may contribute to autoimmune disease and the inflammation that worsens heart failure. They also may play a role in the rejection of transplanted organs, especially the xenotransplantation of organs from pigs to humans, an important advance that is growing closer to clinical testing at UAB.
“Despite the critical role that cells in the immune system play in these disease processes,” said Frances Lund, Ph.D., “we know remarkably little about human immune cells that reside in tissues.”
“At least six of the top 10 causes of death in the United States can be attributed — at least in part — to dysregulated or impaired immunity,” said Lund, the chair of Microbiology at UAB and principal investigator of the National Institutes of Health grant. “These top 10 killers include diseases that are exacerbated or caused by inflammation in tissues like the heart for cardiovascular disease, the brain for Alzheimer’s disease, and the lung for COPD and asthma. They also include diseases exacerbated or caused by failure of immune surveillance or immune protection in tissues that are either infected or contain malignant cells.”
From mouse experiments and human experiments with easily reached tonsils, researchers know that immune cells that are permanent residents in tissues or spend most of their time in organs are very different from immune cells that travel through the blood or lymphoid tissues. They differ in their lifespans, functions, abilities to proliferate and roles in effecting an immune response. However, most human tissues that harbor permanent resident-immune cells are inaccessible.
 Gifts of hope
Gifts of hope
So, the challenge for study of human tissue-resident B cells is how to reach tissues that include bone marrow, the spleen, lymph nodes, the lungs, the small intestine and the omentum, a fatty curtain of tissue that drapes across the abdomen.
The solution involves gifts of life, as families donate organs from brain-dead loved ones for transplantation and donate other vital tissues for research that improves medical care and cures.
UAB works closely with the Birmingham-based Legacy of Hope, Alabama’s organ and tissue donation alliance, formerly known as the Alabama Organ Center. Legacy of Hope evaluates and recovers deceased donor organs for transplant. As part of donors’ research consent, it will recover tissue for the fundamental study of human immunology by Lund and a highly accomplished interdisciplinary group of UAB colleagues.
Legacy of Hope is one of only nine stand-alone centralized organ donation centers in the United States, and it is the only one associated with a research-intensive academic medical center with a strong immunology research community.
Legacy of Hope’s primary mission is to provide organs and tissues for transplant. Legacy of Hope also recognizes the importance of research and the impact it can make on society. “We are pleased we can support a project of this nature due to the generosity of our donors and their families,” said Chris Meeks, executive director Legacy of Hope.
“Through our partnership with the center,” Lund said, “the program will receive primary matched human tissue samples with minimal ischemic damage that cannot be obtained in the same volume or with the same precision in any other institution.”
Three projects
Lund expects to study tissue from 90 to 100 donors over the five-year grant from the National Institute for Allergy and Infectious Diseases. B cells and antibody-secreting cells isolated from the tissues will be carefully catalogued and frozen for further study in three projects.
The first project will study the poorly understood mechanisms that drive development of innate-like B cells, including where are they are located, the traits they exhibit and the antigens they react against. Understanding these mechanisms will help predict susceptibility to serious infections by bacteria like streptococci and pathogenic E. coli. It also will allow development of ways to prevent chronic allergy and autoimmune disease.
The second project will look at human flu-specific B cells and antibody-secreting cells that reside in tissues. Understanding how they contribute to anti-viral immunity will allow rational design of vaccines to elicit the most protective types of memory B cells in appropriate tissues and organs. In recent winters, between 12,000 and 80,000 Americans have died from influenza and its complications, and the effectiveness of vaccination decreases as people age.
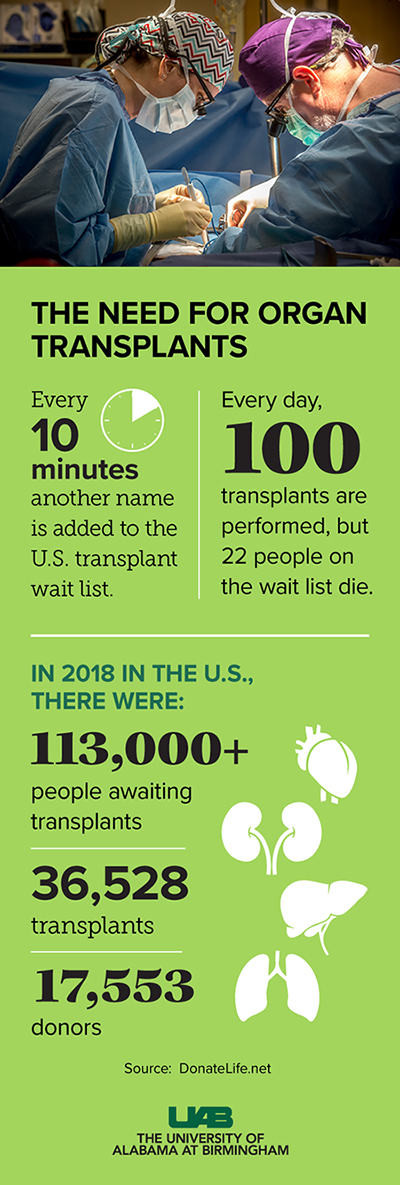
The third project will identify B cell populations that contribute to xenograft rejection, particularly B cells specific for swine leukocyte antigens. Xenografts of pig organs into humans would help the more than 1,800 people awaiting lifesaving transplants through Legacy of Hope. Nationwide, the number awaiting transplants is more than 113,000.
The third project will also develop tools to rapidly screen blood of people on the transplant list to identify swine leukocyte antigen reactivity. Although three well-defined carbohydrates that cause xenograft rejection have been removed from genetically engineered pigs, half of humans still have antibodies to lesser-defined antigens that could lead to transplant rejection.
Co-leaders of the first project are John Kearney, Ph.D., distinguished professor of microbiology at UAB, who holds the Endowed Professorship in Immunology, and Rodney King, UAB assistant professor in microbiology. Second project co-leaders are Lund and Troy Randall, Ph.D. At UAB, Lund holds the Charles H. McCauley Chair of Microbiology, and Randall holds The Meyer Foundation William J. Koopman, M.D., Endowed Professorship in Immunology and Rheumatology in the UAB Department of Medicine. The third project leader is Joseph Tector, M.D., Ph.D., a transplant surgeon who is professor of surgery at UAB and founding director of the UAB Xenotransplantation Program.
More than 600-page application
Lund and colleagues first saw the NIH request for applications on Dec. 17, 2017. The due date was the end of March 2018.
Randall, Lund, Tector and Kearney immediately called all hands on deck in their four labs, putting 20 researchers to work full time on preliminary experiments for the U19 grant application. In three and a half months, they demonstrated their ability to collect donor tissues, isolate good numbers of B cells and antibody-secreting cells from the tissues, and analyze the genetic and molecular details of those cells.
Lund calls it a bucketload of work.
The grant application — totaling more than 600 pages — was written in three weeks. Lund and other leaders cited invaluable help from UAB administrative staff, especially the leadership of Carol Ballinger, Ph.D., administrative director of the Comprehensive Arthritis, Musculoskeletal, Bone and Autoimmunity Center, and UAB microbiology administrative team members Kristina Sinclair, Huantao “Megan” Lu and Jonathan Averett. Also, Cathy Tarver, MBA, the grants and contracts senior officer for federal submissions in the UAB Office of Sponsored Programs, was invaluable in helping submit the grant application to NIH.
The grant was awarded late last year, and it began this month.
“We are in a lot of meetings right now to get queued up to actually start doing experiments,” Lund said. Besides the project leaders, partners in the tissue collection will be Jayme Locke, M.D., the UAB transplant surgeon who directs the UAB Comprehensive Transplant Institute, and Sam Windham, M.D., the UAB surgeon who is critical care specialist for the Legacy of Hope.
 John Kearney, Ph.D.Two key cores for the research will be the tissue collection and repository core and the data analytics and bioinformatics core. The repository core will be led by Matt Tector, Ph.D., assistant professor in the Division of Transplantation in the UAB Department of Surgery; Louis Bridges, M.D., Ph.D., the Anna Lois Waters Professor and director of the Division of Clinical Immunology and Rheumatology in the UAB Department of Medicine; and Anoma Nellore, M.D., assistant professor in the UAB Division of Infectious Diseases and associate medical director, UAB Transplant Infectious Diseases. The bioinformatics core will be led by Alex Rosenberg, Ph.D., associate professor in the UAB Department of Microbiology and member of the UAB Informatics Institute.
John Kearney, Ph.D.Two key cores for the research will be the tissue collection and repository core and the data analytics and bioinformatics core. The repository core will be led by Matt Tector, Ph.D., assistant professor in the Division of Transplantation in the UAB Department of Surgery; Louis Bridges, M.D., Ph.D., the Anna Lois Waters Professor and director of the Division of Clinical Immunology and Rheumatology in the UAB Department of Medicine; and Anoma Nellore, M.D., assistant professor in the UAB Division of Infectious Diseases and associate medical director, UAB Transplant Infectious Diseases. The bioinformatics core will be led by Alex Rosenberg, Ph.D., associate professor in the UAB Department of Microbiology and member of the UAB Informatics Institute.
A third key core is the B-cell-receptor repertoire, cloning and expression core. Co-leader Randall has developed tools to identify, track and characterize antigen-specific B cells, and co-leader King has developed a high-throughput platform to accelerate analysis of B-cell receptors expressed by specific subsets of antigen-specific B cells. This core will identify clones of B cells in various tissues, determine their relatedness to one another, and define their antigen-binding properties and effector functions.
This new interdisciplinary grant fits goals of UAB’s strategic plan, Forging the Future. These include the culture of collaboration and innovation at UAB. They also include efforts to meet grand challenges, such as the School of Medicine’s research focus on inflammation, infection and immunity, or I3. I3 impacts almost all the diseases studied and treated at UAB, and UAB has responded with unified, interdisciplinary programs in fundamental and clinical inflammation-based research.
