 Researchers at the University of Alabama at Birmingham have identified a potential new pathway to treating radiation-resistant glioblastoma, one of the most aggressive forms of brain cancer. The research, performed in animal models and human and mouse cells in culture, was published in the Journal of Clinical Investigation. The findings indicate that an adhesive cell surface protein known as N-cadherin — or N-cad — may be key in overcoming glioblastoma’s resistance to radiation therapy.
Researchers at the University of Alabama at Birmingham have identified a potential new pathway to treating radiation-resistant glioblastoma, one of the most aggressive forms of brain cancer. The research, performed in animal models and human and mouse cells in culture, was published in the Journal of Clinical Investigation. The findings indicate that an adhesive cell surface protein known as N-cadherin — or N-cad — may be key in overcoming glioblastoma’s resistance to radiation therapy.
Glioblastoma is the most aggressive brain tumor. Even with the best surgery, radiation and chemotherapies, patients’ survival is less than two years on average. While radiation is a frequently used therapy to kill tumor cells, medical scientists have long known that, in glioblastomas, some tumor cells are resistant to radiation. Those surviving cells continue to reproduce and spread throughout the brain, leading to tumor regrowth.
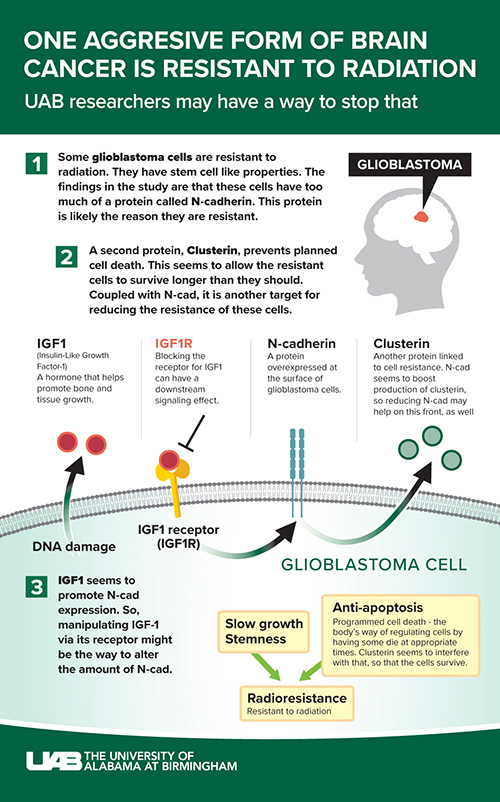
The surviving tumor cells have stem cell-like properties, and are referred to as glioma stem cells, or GSCs. They are innately less sensitive to radiation than the rest of the tumor. The UAB-led research team found that resistant GSCs are more adhesive and cluster together because they express increased levels of N-cad protein, which augments their resistance.
“Prior studies have revealed that radiation-resistant glioma cells behave like stem cells, they grow slowly but can multiply very efficiently, properties that contribute to resistance,” said Erwin G. Van Meir, Ph.D., professor in the Department of Neurosurgery, associate director of the O’Neal Comprehensive Cancer Center and the study’s senior author. “Currently, there are no effective therapies to target radiation-resistant GSCs, so deciphering the molecular pathways that underlie resistance is critical to develop new effective therapies for glioblastoma.”
“Our study reveals a major role for N-cad in establishing radiation resistance in mouse and human GSCs and further shows that lower N-cad levels correlate with glioblastoma patient survival,” said Satoru Osuka, M.D., Ph.D., assistant professor in the Department of Neurosurgery and first author of the paper. “Direct transfer of N-cad to sensitive GSCs made them resistant, and knockout of N-cad sensitized them to radiation, showing therapeutic potential. Altogether, these data indicate that N-cad is a driver of tumor resistance to radiation therapy, and provide proof-of-principle that targeting N-cad might sensitize the tumor to radiation, a major goal in oncology.”
There is also another player at work, a glycoprotein called Clusterin, or CLU for short. CLU is normally secreted in response to stress and regulates cell survival, playing an important role in anti-apoptosis (programmed cell-death) signaling in cancer. It is overexpressed in several cancers, including malignant glioma.
“Our studies now reveal a new role for Clusterin in GSC resistance, and establish a novel relationship between CLU and N-cad expression,” Van Meir said. “We found that N-cad is a strong inducer of CLU expression in GSCs, thereby inducing an anti-apoptotic state through elevation in CLU secretion. This innovative finding provides a new avenue for targeting the N-cad/CLU survival signaling axis to reduce radiation resistance in GBM.”
Clusterin inhibitors are now being analyzed in clinical trials, and this study provides the rationale for testing them in glioblastoma in conjunction with radiation therapy to prevent the emergence of resistance.
The team also found that upregulation of N-cad was induced by radiation-induced IGF1 secretion. IGF1, or insulin-like growth factor 1, stimulates the growth of many cell types by binding to its cell surface receptor. The study shows that IGF1 is significantly involved in radiation resistance via N-cad. Targeting IGF1 could be a way to reduce N-cad expression.
Picropodophyllin, or PPP, is an experimental cancer drug being investigated in several clinical trials. It is a clinically applicable, blood-brain-barrier permeable inhibitor of the IGF1 receptor. Interfering with the receptors that cells use to connect and communicate can disrupt the effects of that communication — in this case, the ability of IGF1 to boost N-cad expression.
 Satoru Osuka, M.D., Ph.D., assistant professor in the Department of Neurosurgery, and Erwin G. Van Meir, Ph.D., professor in the Department of Neurosurgery, associate director of the O’Neal Comprehensive Cancer Center“Clinical trials with an IGF1 receptor inhibitor such as PPP could potentially lead to effective combination therapy for glioblastoma patients,” Van Meir said. “Prior trials using IGF1 receptor inhibitors in glioblastoma patients demonstrated safety; however, they were only tested in recurrent patients who did not receive radiation therapy. Our preclinical data in mice demonstrate that the combination of IGF1 receptor inhibitor and radiation therapy remarkably suppresses radiation resistance of GSC-derived tumors, providing the rationale for testing this combination in new clinical trials for glioblastoma patients.”
Satoru Osuka, M.D., Ph.D., assistant professor in the Department of Neurosurgery, and Erwin G. Van Meir, Ph.D., professor in the Department of Neurosurgery, associate director of the O’Neal Comprehensive Cancer Center“Clinical trials with an IGF1 receptor inhibitor such as PPP could potentially lead to effective combination therapy for glioblastoma patients,” Van Meir said. “Prior trials using IGF1 receptor inhibitors in glioblastoma patients demonstrated safety; however, they were only tested in recurrent patients who did not receive radiation therapy. Our preclinical data in mice demonstrate that the combination of IGF1 receptor inhibitor and radiation therapy remarkably suppresses radiation resistance of GSC-derived tumors, providing the rationale for testing this combination in new clinical trials for glioblastoma patients.”
“Altogether, our collaborative study deepens our understanding of adaptive radiation resistance during repeated irradiation in glioblastoma, and validates the IGF1/N-cad/CLU signaling axis as a novel target for radio-sensitization, which has direct therapeutic applicability,” Osuka said.
This work was supported in part by grants from the NIH (CA163722, NS096236, NS117666, CA212755 and CA223976), Department of Defense (CA170948), the Southeastern Brain Tumor Foundation and the Japan Society for the Promotion of Science (26830082 and 13J06657).
Other authors from UAB are G. Yancey Gillespie, Ph.D., Chaoxi Li, M.D., Christian T. Stackhouse and Christopher D. Willey, M.D. Co-authors from Emory University are Dan Zhu, Zhaobin Zhang and Jeffrey J. Olson. Co-authors from Keio University, Tokyo, Japan, are Oltea Sampetrean and Hideyuki Saya.
