Media contact: Bob Shepard
 UAB has pioneered use of social media to inform the public about an EFIC trial. Imagine you become a trauma patient. It can happen to anyone. You could get in a car accident on the way to work. You could be struck by a stray bullet at a gas station. You could fall off a ladder while working on your home.
UAB has pioneered use of social media to inform the public about an EFIC trial. Imagine you become a trauma patient. It can happen to anyone. You could get in a car accident on the way to work. You could be struck by a stray bullet at a gas station. You could fall off a ladder while working on your home.
Next, imagine that local medical researchers are conducting a trauma research study, and your injury makes you eligible. The focus of the study is an intervention that could increase your chance of survival. The study could ultimately determine whether the intervention should become the standard of care for trauma patients like you, and thousands of lives could be saved.
But you are bleeding severely. You may be unconscious. First responders cared for you at the scene. Doctors are working on you in the emergency department. You are a good candidate for their trauma study. But you are incapacitated and cannot give consent to be part of the trial.
So how do researchers advance the fields of trauma and emergency medicine when dealing with a population that cannot give consent, like those suffering from traumatic injury, stroke or cardiac arrest?
Enter a special kind of trial: the Exception from Informed Consent, or EFIC, trial.
EFIC trials are used in instances of a life-threatening illness or injury when the patient population being studied is unconscious or too sick to provide written or verbal informed consent to be enrolled in a trial.
The federal rules for EFIC trials were developed in 1996. EFIC studies are held to the highest ethical standards and undergo scrutiny by multiple agencies. Researchers must show there is reasonable evidence the study has the potential to provide real and direct benefit to the patient. The study must hold the premise that the new treatment is better than what is currently available.
Patients receive standard treatment in addition to the research treatment, and the research treatment has to have shown promise in earlier or smaller studies.
EFIC at UAB
 Earlier EFIC trials led to the placement of defibrillators in public settings. One of the first EFIC trials conducted at the University of Alabama at Birmingham looked at whether training volunteers in CPR and having automated external defibrillators, or AEDs, available in public areas would affect survival rates for patients with out-of-hospital cardiac arrests. Prior to that trial, which started in 2000, that specific patient population had not been the focus of research.
Earlier EFIC trials led to the placement of defibrillators in public settings. One of the first EFIC trials conducted at the University of Alabama at Birmingham looked at whether training volunteers in CPR and having automated external defibrillators, or AEDs, available in public areas would affect survival rates for patients with out-of-hospital cardiac arrests. Prior to that trial, which started in 2000, that specific patient population had not been the focus of research.
“For 30 years, we never saw an increase in the out-of-hospital cardiac arrest survival rate,” said Shannon Stephens, executive director for UAB’s Center for Injury Science, which conducts multiple EFIC trials. “Because we were able to study CPR and AED use through EFIC, we saw a near doubling of the survival rate. Without EFIC trials, advancing clinical care in trauma and emergency medicine wouldn’t be possible.”
Now, AEDs are widespread and easily accessible in public spaces like offices, schools, airports and stores.
Innovation in informing the community: from town halls to social media
Before an EFIC trial is started, the United States government requires that participating institutions conduct a process of “community consultation and public disclosure.” The goals are to educate community members, invite them to ask questions and give their feedback, and give them the chance to “opt out” of trauma research. Opting out involves getting a wrist bracelet to wear in case the patient receives a trauma injury.
“We take very seriously our obligation to engage community members to get their feedback and input,” Stephens said. “This is a partnership between the community and the institution, and with any kind of partnership, you need to hear from both sides.”
Researchers have to meet a burden of proof, determined at UAB by UAB’s Institutional Review Board, that they have informed enough of the potentially affected population. UAB, as the only American College of Surgeons-verified Level I Trauma Center in Alabama, has a wide enough catchment area to span at least 16 counties and even other states, if the injury is severe enough.
Previously, these community consultations were high-effort and low-payoff, according to Stephens. He says a lot of other trauma centers nationwide will not pursue EFIC trials due to the costly and labor-intensive work of informing the community.
“That’s part of why we haven’t seen the advances in trauma care that we need to, because more sites aren’t willing to do that kind of research,” Stephens said.
UAB has done that work, and put in hours and thousands of dollars to reach the community. Stephens has seen firsthand the low return on investment in many cases: going to town hall forums and Boy Scout meetings, and setting up booths at malls for hardly any face time with community members. Spending thousands of dollars to buy billboards and TV, radio and newspaper ads with no way to track exactly how many people had seen the information.
But with a method pioneered by Stephens, community consultations and public discourse can now take place online through targeted social media ads and webpages. Specific populations can be reached — like people at a higher risk for car crashes who live in commuting areas, people who live in areas with high gun violence, and older people who may be likely to be on an anticoagulant and therefore more likely to bleed after a fall.
And it works
The resulting data is ultra-specific — how many people saw the ad, how many people clicked on the ad, how many people read the informational webpages and for how long. And those people can be broken down by age and gender.
The first EFIC trials at UAB using social media for community consultation focused on one trial studying continuous chest compressions for out-of-hospital cardiac arrests and another that looked at crystalloid fluid resuscitation for hemorrhagic shock.
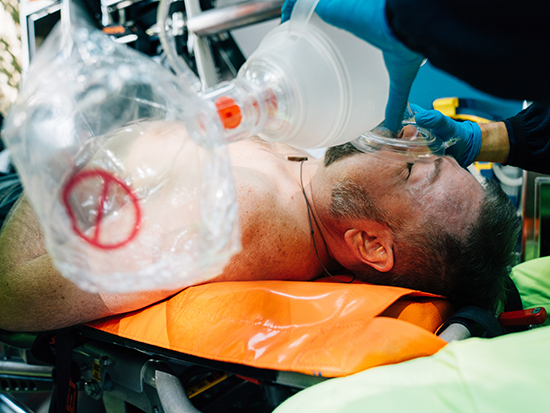 EFIC trials are permitted in situations where a patient is incapacitated and cannot give consent.Stephens determined the areas and demographics he wanted to reach, and built the Facebook ads and websites.
EFIC trials are permitted in situations where a patient is incapacitated and cannot give consent.Stephens determined the areas and demographics he wanted to reach, and built the Facebook ads and websites.
“We were blown away by the results,” Stephens said. “UAB’s IRB was very receptive of this quantifiable data.”
Compared to a previous in-person campaign, which cost $8,000 and reached 465 participants, the social media campaigns resulted in more than 1,000 webpage views for just $1,000 each. The ads for the social media campaigns garnered millions of views.
Stephens published his findings in 2016 and established UAB as a leader in this innovative type of community consultation. UAB has since been the lead center on a number of multicenter EFIC trials and has created the ads and webpages for the other centers.
“We have figured out a way to streamline the process, make it better and make executing these types of clinical trials easier,” Stephens said. “That’s what we’re really excited about.”
“No stone left unturned”
The therapies that are used in trauma clinical trials are already well-vetted by the FDA and proven to work — in certain situations and populations. EFIC trials examine whether the same intervention or medicine will work in another subset of patients.
An example: Stephens says tranexamic acid was previously not used in the pre-hospital setting but was proved to help treat patients with hemorrhagic shock and traumatic brain injury once patients arrived at the hospital. After favorable findings from an EFIC trial, TXA is now available on most ambulances in Alabama.
UAB Hospital currently holds a 96 percent survival rate for trauma patients, and Stephens says UAB is always striving to improve the care it provides.
“If it were my family member or loved one in a trauma situation, I would want no stone left unturned,” Stephens said. “I would want to give them every opportunity to survive.”
Stephens was a paramedic for 30 years. He says he is “still a paramedic at heart,” but he finds research fulfilling.
“Instead of providing care one patient at a time, I see an opportunity to really advance trauma care delivery in a way that can impact thousands or tens of thousands of lives a year,” Stephens said.
The Center for Injury Science was founded in 1999 and conducts research to improve trauma care. The mission of CIS is to promote injury prevention and to improve outcomes from injury at all stages of care, from the pre-hospital setting through to resuscitation, acute care and rehabilitation. Clinicians from many specialties — including trauma surgeons, emergency medicine physicians, anesthesiologists, intensivists and rehabilitation specialists — work closely with epidemiologists, basic scientists, biostatisticians, health economists, health psychologists and methodologists. Learn more about current EFIC trials taking place at UAB here. Opt out of trauma research here.
