Reporter Staff
| This email address is being protected from spambots. You need JavaScript enabled to view it.How did we do? Forging the Future of Research
How did we do? Forging the Future of Education
There are no approved treatments specifically for cocaine use disorder. We are looking for adults (18-65 years of age) who are using cocaine and who are interested in reducing or stopping their use. The purpose of our study is to test a combination of two medications for 2 months as a possible treatment for cocaine use disorder.
This study includes the use of a placebo, which looks like the medication we are testing but does not include any active medicine. Study medication and assessments will be provided to participants at no cost. Participants will receive compensation for their time and efforts.
Study information will be kept strictly confidential. Your privacy is important to us.
Call us for more information at 205-996-0211 or email us at curb2study@uabmc.edu
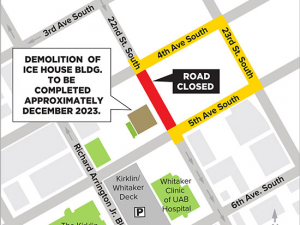
Wellscreens appointments now available for fall — register today
Where to buy UAB merch so you can ‘Be Seen in Green’
UAB employees and fans are encouraged to wear green each Friday to show their support for UAB Football and the Blazer community. Wondering where to get your UAB gear? Check out a list of places on campus, online and around town.