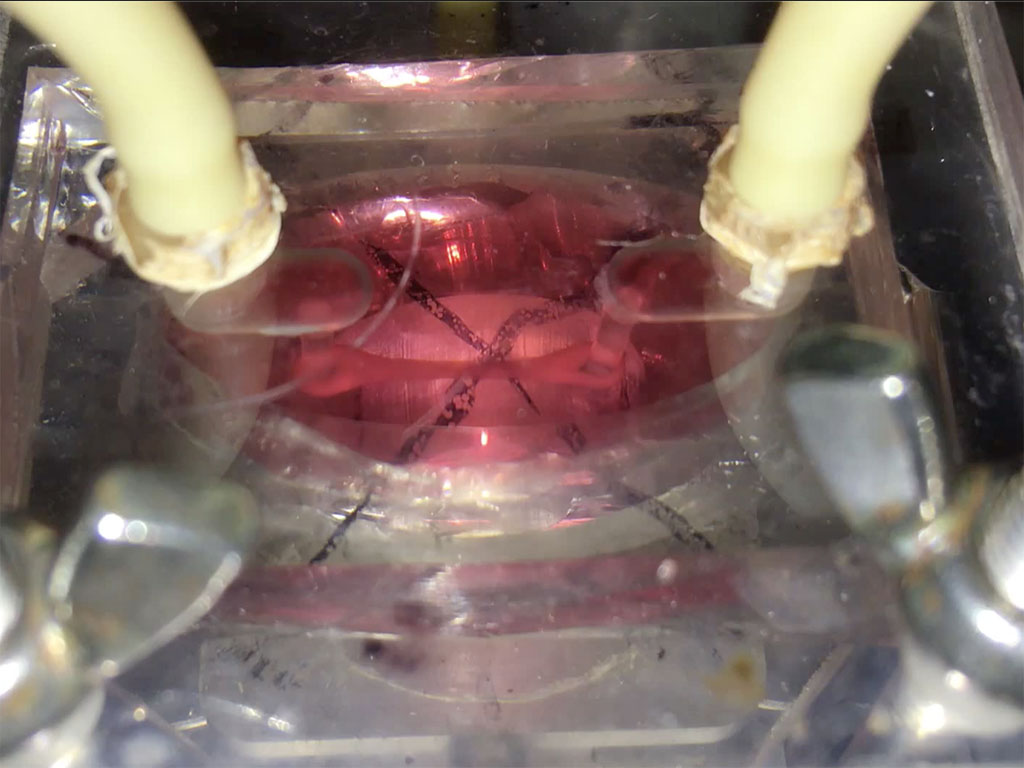
Putting a heart through its paces: Watch a tiny sliver of human heart tissue stretch and contract in this video from Dr. Palaniappan Sethu’s lab.The heart of the heart is the beat. It’s a complex, coordinated mix of stretching, contraction and pressure that can go wrong in a number of ways. But by getting it right, UAB researchers hope to open a new era in tissue engineering.
Putting a heart through its paces: Watch a tiny sliver of human heart tissue stretch and contract in this video from Dr. Palaniappan Sethu’s lab.The heart of the heart is the beat. It’s a complex, coordinated mix of stretching, contraction and pressure that can go wrong in a number of ways. But by getting it right, UAB researchers hope to open a new era in tissue engineering.
Developing a viable model of human cardiac tissue is a pressing concern. Up to 30% of new drugs fail because they turn out to be toxic to humans; 60% are rejected because they are not as efficacious in people as they were in animal models. Then again, many drugs that are successful in humans would not have shown promising results in animal models. “Aspirin would fail almost all safety tests and if you gave aspirin to rats at the maximal allowed doses for humans, more than 50% of them would die,” said Palaniappan Sethu, Ph.D., director of the Cardiovascular Bioengineering Lab and associate professor in the Division of Cardiovascular Disease and Department of Biomedical Engineering. “How many drugs that would have worked well in humans have we missed?”
One of the primary drug toxicities seen in humans is an increased risk of arrhythmias. This is exactly what early trials have found when patients with coronavirus have received the malaria drug hydroxychloroquine. “Any drug can interact with ion channels in cells and cause arrhythmias that can often be fatal,” Sethu said. Heart muscle cells — cardiomyocytes — are particularly vulnerable because they have lots of ion channels that these drugs can interact with.
 Palaniappan Sethu, Ph.D., director of the Cardiovascular Bioengineering Lab and associate professor in the Division of Cardiovascular Disease and Department of Biomedical Engineering.Organs on a chip — lab-grown human cells that offer a promising way to test new drugs, model disease and generate tissues for transplant, among other uses — have come a long way in the past decade. One of the biggest leaps forward came with the development of induced pluripotent stem (iPS) cells. Add a magic mixture of four transcription factors to human skin cells in a lab dish and you can turn them into stem cells capable of producing virtually any specialized cell type in the body. That includes heart muscle cells, which have proven to be some of the most difficult to develop in the lab.
Palaniappan Sethu, Ph.D., director of the Cardiovascular Bioengineering Lab and associate professor in the Division of Cardiovascular Disease and Department of Biomedical Engineering.Organs on a chip — lab-grown human cells that offer a promising way to test new drugs, model disease and generate tissues for transplant, among other uses — have come a long way in the past decade. One of the biggest leaps forward came with the development of induced pluripotent stem (iPS) cells. Add a magic mixture of four transcription factors to human skin cells in a lab dish and you can turn them into stem cells capable of producing virtually any specialized cell type in the body. That includes heart muscle cells, which have proven to be some of the most difficult to develop in the lab.
“Because the heart has very limited capacity to regenerate, you really can’t isolate heart muscle cells from humans,” Sethu said. “The only ones available are from failed transplant hearts, but there are complexities associated with those. Now, with iPS cells you have a human option and in theory you can propagate them indefinitely.”
The iPS approach is great at turning skin cells or blood cells into immature cardiomyocytes, Sethu explained. The trouble is, these immature cells are a far cry from the final product seen in actual humans. The heart starts out as a hollow, pulsating tube. Then, during embryonic development, “it bends around and forms this complex, four-chamber organ,” Sethu said. “What really drives this is a combination of electrical and mechanical stimulation — if you want to mature these cells, putting them in a dish is not going to get you there. But so far, nobody wants to build models with enough complexity to accurately depict what’s happening in the body.”
That’s where Sethu and his team come in. They have built something called the biomimetic cardiac tissue model — a miniature chamber that can put immature cardiac cells through the pressure and stretch conditions that induce them to develop into mature heart tissue. “There have been lots of studies where researchers differentiate these cells and put them in a heart and their survival is very poor,” Sethu said. “They haven’t been exposed to the pressure and stretch conditions that would allow them to tolerate that harsh environment.”
The biomimetic cardiac tissue model does more than simulate normal conditions; it can also re-create deterioration caused by hypertension and other disease states.
This spring, Sethu received a four-year R01 grant from the National Heart, Lung, and Blood Institute to develop the technology behind the biomimetic cardiac tissue model. The grant, titled Tissue Chip Models for Cardiovascular Development and Disease, has three projects, he explained. The first is to develop methods to transition cardiac cells from an immature to a mature phenotype. The second will explore ways to take diseased tissue back to normal function. The third will examine ways in which the biomimetic cardiac tissue model affects the circadian clocks inside cardiac cells. “During the day you see slightly higher pressures, for instance, and during the night the heart rate goes down significantly,” Sethu said. “We’re going to see if changing these parameters impacts the circadian clocks.”
Sethu began his cardiac tissue model while at the University of Louisville, where he collaborated with the lab of Sumanth Prabhu, M.D., who now is director of the Division of Cardiovascular Disease at UAB. “Dr. Prabhu suggested there were not too many physiologically relevant in vitro models of the heart; I had a collaborator, Dr. Guruprasad Giridharan, who built flow loops for testing heart pumps, and we had expertise in three-dimensional tissue engineering,” Sethu said. “We put all that together, and that has been the basis for our work.” During the past eight or nine years, the chip has evolved to the point where “we are close to a final version,” Sethu said. “With this grant we aim to accomplish that; our chips would probably be the state of the art. With this funding we can scale everything up.”
The cardiac chip isn’t the only model under development in Sethu’s lab. “We have a vascular chip model and a student is working on a renal chip model,” he said. “The NIH is really pushing this idea of getting multiple chips to talk to each other. Right now, if you want to go from these [individual] chips to patients it’s a leap too far. Integrating multiple chips brings us a step closer.”
The biggest challenge will be to find a way to integrate the immune system into a multi-chip model, Sethu said. “We’ve started working on incorporating the resident immune cells in the tissue,” he said. “We’re also looking at creating a secondary lymphoid organ which would be capable of mobilizing immune cells to the tissue location.”
Tissue-chip models are like a big jigsaw puzzle, Sethu explained. “At UAB, we have assembled a team of experts from cardiology — Drs. Prabhu, Tariq Hamid and Martin Young — and Biomedical Engineering — Drs. Jay Zhang, Vladimir Fast and Ramaswamy Kannappan — to model cardiovascular development and disease. You can’t solve the whole thing at once — you put little pieces together and then build up from there.”