Health technology, like consumer electronics, is constantly evolving. Each year, companies develop devices they hope will achieve the widespread use of now-standard technology, such as electronic pacemakers for heart conditions or digital insulin pumps for diabetes.
Before they can receive approval for marketing in the United States, these new devices must show effectiveness in human clinical trials. And in many cases, these trials come to UAB, where Alabamians benefit from early access to promising technology.
Here, we explore a few of the dozens of device trials currently enrolling participants at UAB. (Find more clinical trials at UAB here and on the UAB Reporter's clinical trials page.)
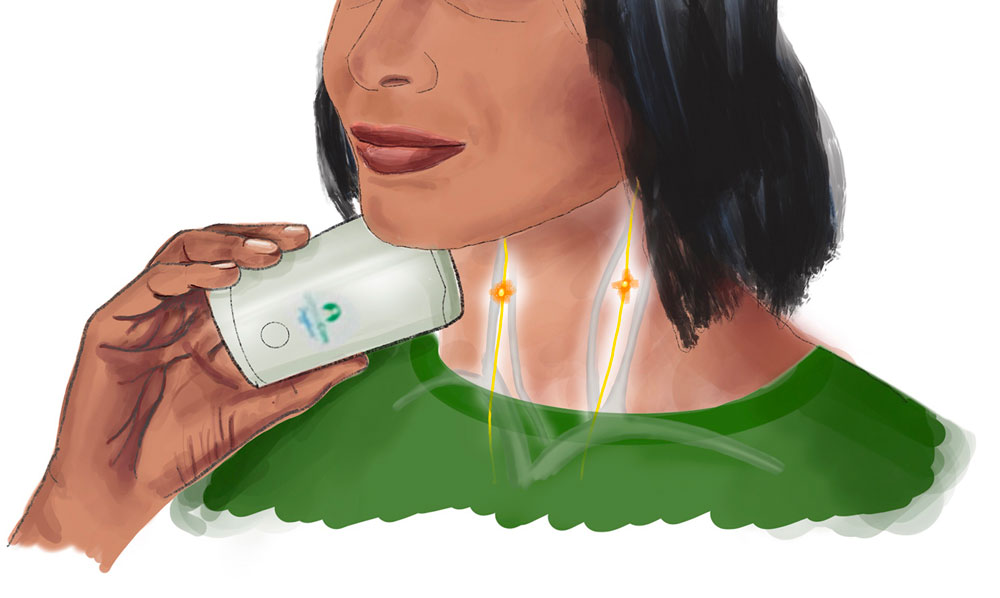 Illustration by Jody Potter | University Relations
Illustration by Jody Potter | University RelationsNon-invasive vagal nerve stimulation (nVNS)
Depression
Matthew Macaluso, D.O., clinical director of the UAB Depression and Suicide Center, Bee McWane Reid Professor and vice chair for Clinical Affairs in the Department of Psychiatry and Behavioral Neurobiology in the Heersink School of Medicine, is principal investigator for the Vagal Nerve Stimulation for Treatment Resistant Major Depression clinical trial. “Electrical stimulation of the nervous system to modulate or modify function ... has been FDA-approved in the United States since the late 1990s,” trial materials explain. Stimulation of the vagus nerve through a device implanted in the patient (implantable VNS, or iVNS) “has been approved for use in epilepsy and depression.” Because previous studies have shown that patient response increases over time, and continues for up to five years with continued stimulation, researchers believe that “VNS gradually changes brain function through neuroplasticity.”
Macaluso’s trial is studying the antidepressant effects of a non-invasive technology, gammaCoreTM, over a period of three months in study participants. Researchers will measure the change in severity of depression ratings over that period, as well as any adverse events. The trial is open to people ages 18-75 with a Hamilton Depression Rating Scale total score greater than or equal to 18 and a DSM 5 diagnosis of major depressive disorder. See exclusion criteria and other details at the NIH’s ClinicalTrials.gov website.
 Illustration by Jody Potter | University Relations
Illustration by Jody Potter | University RelationsComputerized handwriting training
Tourette syndrome and tic disorders
It is difficult to do well in school, even in these days of laptops in classrooms, without handwriting skills. And even if computers are available, “strong evidence indicate[s] that handwriting has more cognitive and neurological benefits than using a keyboard when taking notes in the classroom,” as materials for this trial note.
The principal investigator is Jan Rowe, DrOT, coordinator of the Tourette Syndrome Clinic at Children’s of Alabama. In a previous study, Rowe demonstrated that children with Tourette syndrome and tic disorders “are more likely to have handwriting deficits ... [and] are also more likely to have challenges in their academic performance and success as evidenced in the literature.”
Practice is the best way to improve handwriting. Rowe’s clinical trial is using a device called a Rocketbook, which allows children to practice handwriting on special paper that is then emailed to Rowe through the device’s app. “Given that the practice is completed on an electronic device, this handwriting program may serve as a strong incentive to motivate children to practice handwriting,” Rowe said. Participants, ages 7-17 years old, must be diagnosed with a tic disorder and/or Tourette syndrome. They practice five to seven days a week, for a minimum of 15 minutes, for eight weeks. See exclusion criteria and other details at the NIH’s ClinicalTrials.gov website.
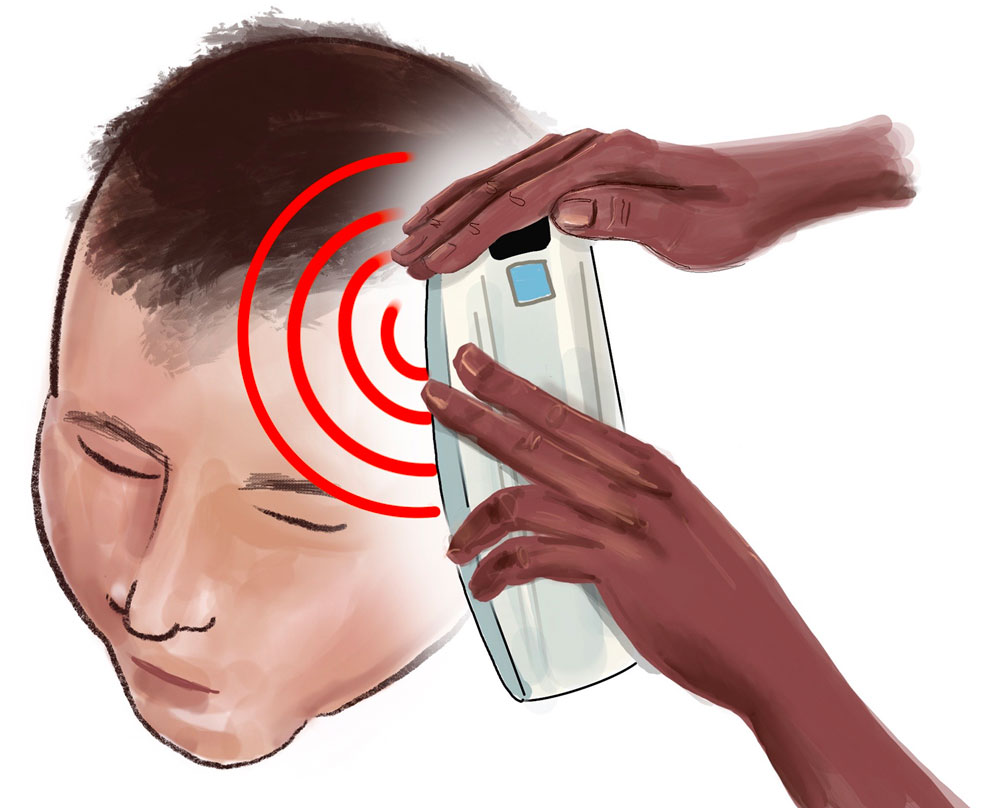 Illustration by Jody Potter | University Relations
Illustration by Jody Potter | University RelationsNon-invasive monitoring of TBI progression
Traumatic brain injury
In this multicenter clinical trial, known as MOBI-1, investigators are evaluating a non-invasive device for monitoring traumatic intracranial hematomas. These form after a head injury (often a car crash or a fall) that results in blood accumulating in the brain, or between the brain and the skull. The portable device, the Infrascanner© 2000, uses near-infrared technology to identify patients who might benefit from immediate referral for a CT scan and neurosurgical intervention to remove the blood and prevent more serious brain damage.
Jan Jansen, MBBS, Ph.D., director of the UAB Center for Injury Science and associate professor in the Department of Surgery’s Division of Trauma and Acute Care Surgery, is the principal investigator for the study. All patients receive standard care in addition to hourly assessments with the Infrascanner. The study will test the hypotheses that the Infrascanner can detect the expansion of intracranial hematomas earlier, and that patients with these expanding intracranial hematomas will be identified faster when monitored with hourly Infrascanner assessments compared to standard care alone.
The study is enrolling participants age 15 or older admitted to one of 10 trauma centers nationwide, including UAB, who have been admitted for observation for a traumatic brain injury in either a step-down unit or the intensive care unit, with a CT scan showing intracranial hemorrhage, a score on the Glasgow Coma Scale less than 15. (The neurosurgery service must also determine that their initial care is nonoperative.) See exclusion criteria and other details at the NIH’s ClinicalTrials.gov website.
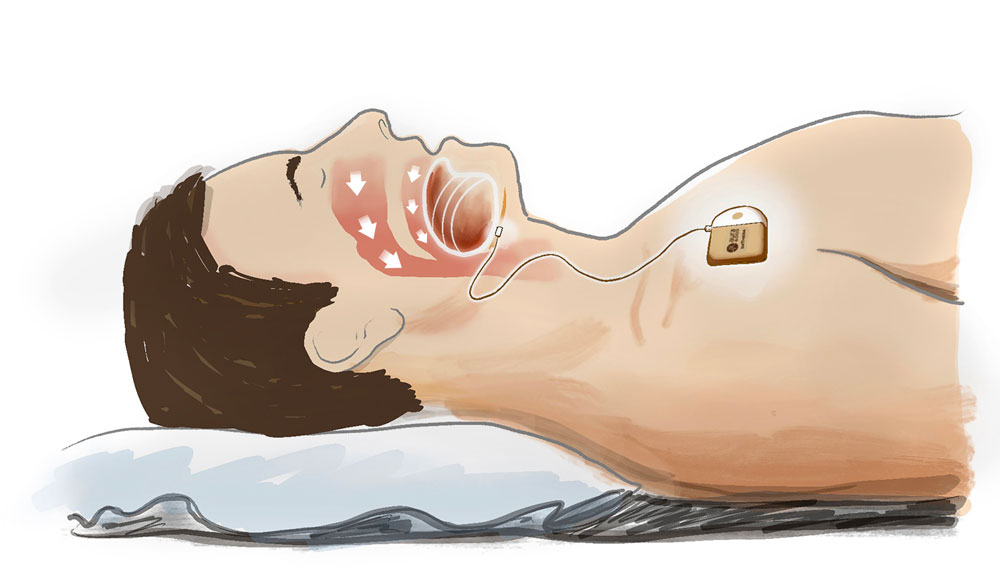 Illustration by Jody Potter | University Relations
Illustration by Jody Potter | University RelationsImplanted hypoglossal nerve stimulator
Sleep apnea
Apnea refers to any period when normal breathing stops, and sleep apnea is the widespread condition in which breathing starts and stops during sleep. Obstructive sleep apnea, the most common type of sleep apnea, is caused by weight on the upper chest and neck blocking the flow of air through a person’s upper airway. It affects one in five American adults, according to the American Heart Association. Moderate to severe obstructive sleep apnea is often treated with a continuous positive airway pressure, or CPAP, device, which uses a mask or mouthpiece to keep the upper airway open and regulate breathing. But CPAP therapy is not effective for some patients, and even more dislike the experience so much that they refuse to use it.
An alternative therapy approach is hypoglossal nerve stimulation, which uses an implanted device to electrically stimulate the nerve running to the tongue from the base of the brain. The device’s stimulation is timed during breaths to move the tongue out of the upper airway to ensure adequate air flows through.
Kirk Withrow, M.D., associate professor in the Department of Otolaryngology, is principal investigator for this trial of the aura6000™ System. Participants will receive hypoglossal nerve stimulation for six months, and researchers will compare their responses (including decreases in oxygen saturation index and changes in sleep) to six months of no therapy. Participants must be age 22 or older with a diagnosis of moderate to severe obstructive sleep apnea. Those who cannot tolerate positive airway pressure therapy or refuse to use it are eligible. See exclusion criteria and other details at the NIH’s ClinicalTrials.gov website.
