 |
| Project
3: Role of Fungi in Chronic Airway Disease and Disseminated Fungal
Infections (A) In the last 25 years, asthma and autoimmune diseases have increased in the industrialized world. The “hygiene hypothesis” relates this increase to a failure of proper microbial stimulation of the immune system due to increased sanitary conditions early in life. Scarlet Fever, caused by the Streptococcus pyogenes bacterium, is inversely correlated with asthma. S. pyogenes induces large amounts of antibody to cell wall-associated N-acetyl glucosamine (GlcNAc), which is also the major component of chitin, a component of many allergen-bearing organisms. We will study the link between innate and adaptive immunity and asthma induced by environmental allergens containing GlcNAc and other conserved carbohydrates. We will quantitate the specificity, clonality, airway association and longevity of the antibody response to S. pyogenes, chitin and Aspergillus fumigatus. We will determine the role of B cells and antibody in modifying innate cell functions and activation of CD4 T cells involved in the chronic inflammatory process and airway obstruction of asthma. Apart from the established paradigm involving the shift in TH1/Th2 functionality and the important but not definitive role of IgE in asthma, B cell and antibody functions in the disease have not been extensively studied. By focusing on the role of B cells and antibodies, we may shed light on the mechanisms involved in the developmental induction of allergic asthma and treatment of established disease. |
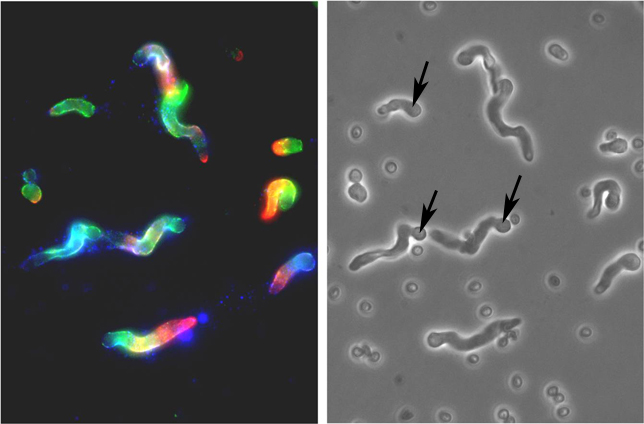 |
| Fig 1. Reactivity
of monoclonal antibodies (Mabs) with A.f. The photomicrographs show
that Mabs raised against bacterial antigens also bind to conidia and
hyphae of A.f. Methods: A.f. conidia on poly-L-lysine coated slides
were incubated for 9 hrs in 10% fetal calf serum and RPMI 1640 medium
at 37C. After fixing in 95% ethanol for 30 min they were stained with
Mabs to alpha-1-3 glucan (green), sialyllacto-N-tetraose (red), GlcNAc
(blue) (left). Phase contrast of the same field with arrows showing the
swollen conidia with growing hyphae (right). |
| (B) Development of Passively Administered Nebulized Antibodies for Prevention of Asthma Natural exposure to aero-allergens responsible for initiating and maintaining allergic airway diseases occurs rarely, if ever, as a single purified allergenic molecule. Rather, potential T cell-sensitizing allergens are cargo, associated with innate immune-activating particulates. Since then in three mouse models involving the common human allergens house dust mites, Aspergillus fumigatus and German cockroach we showed that vaccine-induced antibodies and monoclonal antibodies (mAbs) passively transferred into the lung protect against development of allergic airway disease. The Aim of this extension application is to generate and test panels of human monoclonal antibodies similar to those from mice that react with chitin, glucans, phosphorylcholine, and other conserved allergic organism-associated molecules. Intact human mAbs or their fragments will then be tested for protection in our mouse models. Our observations that intratracheal introduction of mAbs is effective in these models supports the feasibility of our strategy to develop antibodies for blocking allergen-host innate receptor interactions at the air-lung interface with nebulized antibodies. Although few biotherapeutics delivered via the airway have been developed, multiple recent preclinical and pharmacokinetic studies indicate increasing interest in this approach for treating infectious disease, and cancer, as well as allergic disease. Although many unanswered questions must be addressed before this novel approach involving mAbs can be transferred from the bench into respiratory care medicine, our planned isolation and characterization of human mAbs with this potential in a preclinical setting will be a crucial step in this direction. (C) Repurposing of Anti-Bacterial Vaccines for Immunity Against Fungal Infections The morbidity associated with invasive fungal infections (IFIs) is high and IFIs are highly intractable to treatment once these opportunistic pathogens gain a foothold. There is a rapidly rising incidence in hospital-acquired nosocomial infections which are likely have an increasing impact on health care. Compounding the serious nature of IFIs is the relative lack of antifungal drugs, the emergence of triazole resistant fungal strains, one of the few classes of effective anti-fungal agents, and the lack of any licensed anti-fungal vaccine. The long term goal of this project is to provide new active vaccination and passive protection strategies that provide more optimal prevention and treatment for personnel at risk for IFI. We are proposing a novel approach that involves vaccination with immunogens expressing epitopes shared by Aspergillus fumigatus and other fungi with bacteria. This strategy will circumvent the low responsivity of the immune response to surface fungal antigens which normally leading to a lack of protective immunity in fungal infections. This approach, to develop active immunity against IFI, is based on findings that (i) immunization of mice with whole heat-killed strains of streptococci induced protection against invasive aspergillosis and (ii) anti-A. fumigatus Abs are induced in humans by conjugate vaccines that have been used to vaccinate humans against streptococcal infections. Repurposing of prophylactic conjugate vaccines and availability of passive monoclonal antibodies for protection against IFI by multiple fungal species would have a high impact and provide much needed treatments for patients at risk for, or suffering from IFIs. |