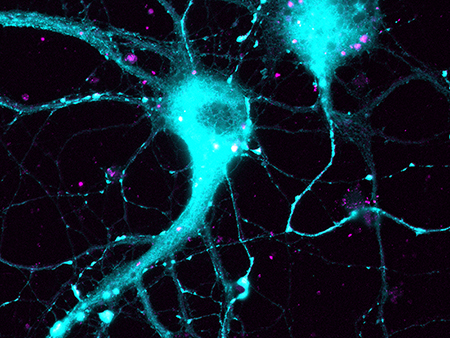
 The neurons in this image are stained blue, indicating the presence of the BIN1 protein. Points of direct interaction between BIN1 and calcium channels are in purple.Researchers at the University of Alabama at Birmingham are on the track of a gene that might play a role in the development of Alzheimer’s disease. The research team is studying a gene called BIN1, which was first linked to Alzheimer’s disease in 2009.
The neurons in this image are stained blue, indicating the presence of the BIN1 protein. Points of direct interaction between BIN1 and calcium channels are in purple.Researchers at the University of Alabama at Birmingham are on the track of a gene that might play a role in the development of Alzheimer’s disease. The research team is studying a gene called BIN1, which was first linked to Alzheimer’s disease in 2009.
In a paper recently published online in eLife, the team shows that BIN1 helps to regulate the activity of neurons. This may be significant, as too much neuronal activity, known as hyperexcitability, is associated with Alzheimer’s disease. BIN1 becomes the first gene to be linked to hyperexcitability as a driver of Alzheimer’s disease.
BIN1 was identified as a risk factor for Alzheimer’s following large scale studies called genome wide association studies, which looked at the genomes of thousands of people with and without Alzheimer’s disease.
“These genetic studies showed that variants of BIN1 were present in many of the study participants who had Alzheimer’s,” said Erik Roberson, M.D., Ph.D., the Rebecca Gale Professor in the Department of Neurology, School of Medicine, and lead author of the study. “The problem was that nobody had a clear idea what BIN1 does in the brain.”
Using different ways of increasing BIN1 and measuring neuronal activity, members of Roberson’s lab found that neurons with higher BIN1 levels fired more often and were more prone to hyperexcitability.
“We think that’s important because hyperexcitability is now recognized as a feature of early Alzheimer’s,” said Roberson, who is director of the UAB Alzheimer’s Disease Center and the Center for Neurodegeneration and Experimental Therapeutics. “The neurons fire too often, which appears to lead to damage.”
Prior studies had linked BIN1 to the Tau protein, which has long been associated with Alzheimer’s as one of the hallmarks of the disease.
“Importantly, we found a key role for Tau in the hyperexcitability caused by BIN1,” said Yuliya Voskobiynyk, a senior graduate student in Roberson’s lab who led the work. “Reducing Tau made neurons resistant to the effects on BIN1 on neuronal hyperexcitability. Along with BIN1 and Tau, a third factor is involved: channels that allow calcium into the neuron, which are important for neuronal firing. We found that calcium channels form a complex along with BIN1 and Tau, and reducing Tau not only blocked neuronal hyperexcitability, but also reduced the formation of this complex.”
 Erik Roberson, M.D., Ph.D.Roberson is quick to point out that this research, conducted in animal models and cell cultures, is very preliminary. Tau is a major research focus for investigators worldwide; but the role of BIN1, and its interactions with Tau and calcium channels, is only starting to be explored.
Erik Roberson, M.D., Ph.D.Roberson is quick to point out that this research, conducted in animal models and cell cultures, is very preliminary. Tau is a major research focus for investigators worldwide; but the role of BIN1, and its interactions with Tau and calcium channels, is only starting to be explored.
“It seems clear that something about this gene has a role to play in Alzheimer’s,” Roberson said. “At this point, we don’t know if that role is driven by too much BIN1 protein, too little, or by more subtle changes in the type of BIN1 being made in people with Alzheimer’s disease.”
Roberson says next steps will include digging deeper into the gene’s normal function within the brain, and then working to understand what happens in Alzheimer’s disease. His lab was already working to develop drugs that would block the binding between Tau and proteins like BIN1 as potential therapies.
“This study helps to establish that there is a connection between Tau, BIN1 and calcium channels,” Roberson said. “But we need to learn more. We need to understand how they bind and how binding affects their function. If we can zero in on the molecular details of these interactions, we may be able to find new targets for intervention.”
The research was supported by the National Institutes of Health grants RF1AG059405, R01NS075487, R01MH114990, T32NS095775 and T32NS061788; the
Alzheimer’s Association; and the Weston Brain Institute.
Co-authors are Jonathan R. Roth, J. Nicholas Cochran, Travis Rush, Jacob S. Mesina, Mohammad Waqas and Rachael Vollmer, of the UAB Center for Neurodegeneration and Experimental Therapeutics, Alzheimer’s Disease Center and Evelyn McKnight Brain Institute; Nancy V.N. Carullo and Jeremy Day, Ph.D., UAB Department of Neurobiology; and Lori McMahon, Ph.D., UAB Department of Cell, Developmental and Integrative Biology.
