Protein Glycosylation
Glycosylation is one of the most common post-translational modifications of proteins. It is estimated that over half of mammalian proteins are glycosyalted. Glycoproteins are vital for a wide range of biological processes including: transporting molecules, production of enzymes, acting as cell attachment-recognition sites, etc. The varying types and structures of glycoproteins allow them to adapt to these diverse function. There are two types of protein glycosylation O-linked and N-linked.
O-linked Glycosylation
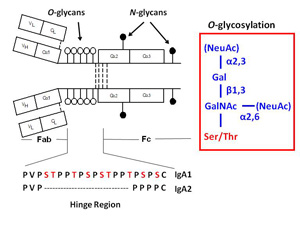
A variety of proteins are postranslationally modified with clustered sites of O-glycosylation. These are serine and threonine rich stretches within the amino acid sequence that have short O-glycan chains. (The picture to the left shows a stretch of clustered O-glyans on IgA1 hinge region.) Some examples of O-glycosylation include the immunoglobulin A (A1 isotype), mucins, and bacterial cell surface proteins. IgAN is an autoimmune disorder that exhibit abnormal glycosylation of serum immunoglobulins (See IgAN). For a given protein with sites of clustered O-glycans, the protein isolated from a single source produces a population of variably O-glycosylated isoforms that we can observe by mass spectrometry. These isoforms usually show a distinct distribution of microheterogeneity in terms of number of chains, the sites of attachment and O-glycan composition at a given amino acid. Characterizing these clustered sites and understanding how the distributions change under different biological conditions or disease states is an analytical challenge that our lab is tackling.
N-linked Glycosylation
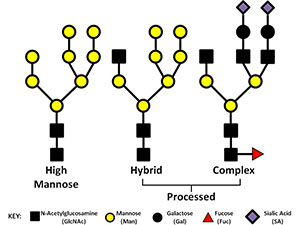
Numerous glycoproteins are N-linked. N-linked glycans are covalently attached to the protein at asparagine (Asn) residues this most often occurs when the new protein is being translated and transported into the ER. Not all Asn can accept an N-glycan. For attachment to occur the amino acid motif usually needs to be Asn-X-Ser/Thr. All N-glycans share a common core sequence, but are further classified into three types (seen in the picture to the right) these are: high mannose, hybrid, and complex glycans. In our research we have been working to elucidate the type and location of the glycans present on IgA1 and gp120 (the envelope glycoprotein of HIV). gp120 presents a tough analytical challenge since the most common gp120 has 25 sites of glycosylation and there can be a myriad of types of glycans present at any given time. Characterizing and locating these sites is important if we want to understand how the envelope mutates and perhaps find sites that could be used for targets for broadly neutralizing antibodies.
Tools for Analyzing Glycoproteins
Primarily through our efforts in understanding the heterogeneity of clustered sites of IgA1 O-glycoslation, our group has developed a series of analytical and bioinformatic tools to assess the site-specific heterogeneity of heavily glycosylated proteins. In addition we have collaborated with software developers to help push MS bioinformatic software platforms that help identify sites of glycosylation, identify low abundance glycopeptides, and define the overall site-specific glycan heterogeneity that allows cross sample comparison.
Collaborators
Our Publications on Protein Glycosylation
We have analyzed a variety of protein from serum, cell culture, recombinant proteins, and in vitro biochemical reactions for both O- and N-type glycosylation.
-
Takahashi K, Raska M, Stuchlova Horynova M, Hall SD, Poulsen K, Kilian M, Hiki Y, Yuzawa Y, Moldoveanu Z, Julian BA, Renfrow MB, Novak J.
Enzymatic sialylation of IgA1 O-glycans: implications for studies of IgA nephropathy.
PLoS One. 2014 Jun 11;9(2):e99026.
- Jordan DS, Daubenspeck JM, Laube AH, Renfrow MB, Dybvig K.
O-linked protein glycosylation in mycoplasma
Mol. Microbiol. 2013 Oct 4. [Epub ahead of print]
With: Mol. Microbiol. 2013 Dec;90(5):1046-53
- Takahashi K, Smith AD, Poulsen K, Kilian M, Julian BA, Mestecky J, Novak J, Renfrow MB.
Naturally occurring structural isomers in serum IgA1 o-glycosylation.
J Proteome Res. 2012 Feb 3;11(2):692-702.
- Takahashi, K., Wall, S.B., Suzuki, H., Hall, S. Smith, A.D.,IV, Poulsen, K., Kilian, M., Julian, B.A., Mestecky, J.,
Novak, J., Renfrow, M.B.,
"Clustered O-glycans of IgA1: Defining macro- and micro- heterogeneity by use of electron capture/transfer dissociation"
Mol. Cell. Proteomics Epub Sep 7. 2010
- Raska, M., Takahashi, K., Hall, S., Moldoveanu, Z., Elliot, Wilson, L., Brown, R., Barnes, S., Tomana, M., Smith, Mestecky, J., Renfrow, M.B., Novak, J.
Glycosylation pattern of HIV-1 gp120 is cell specific and affects binding of neutralizing antibodies.
J. Biol. Chem. Epub May 3, 2010
- Wada, Y., Dell, A., Haslam, S.M., Tissot, B., Canis, K., Azadi, P., Backstrom, M., Costello, C.E., Hansson, G.C., Hiki, Y., ishihara, M., Ito, H., Kakehi, K., Karlsson, N., Koichi, K., Kawasaki, N., Khoo, K.-H., Kobayashi, K., Kolarich, D., Kondo, A., Lebrilla, C., Nakano, M., narimatsu, H., Novak, J., Novotny, M.V., Packer, N.H., Renfrow, M.B., Tajiri, M., Thomsson, K.A., Yu, S.-Y., Taniguchi, N.
Comparison of methods for profiling O-glycosylation: HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1.
Mol Cell. Proteomics. Apr 9(4):719-27, 2010
- Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB.
Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding.
Biochemistry. 28;47(43):11285-99, 2008
- Renfrow, M.B., Mackay, C.L., Chalmers, M.J., Julian, B.A., Mestecky, J., Kilian, M., Poulsen, K., Emmett, M.R., Marshall, A.G., Novak, J.
Analysis of O-glycan Heterogeneity in IgA1 Myeloma Proteins by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry: Implications for IgA Nephropathy.
Anal. Bioanal. Chem. 389:1397-407, 2007.
- Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J.
Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry.
J Biol Chem. 280:19136-19145, 2005.
Our Publications on Glycosyl-transferases
Additionally, we are interested in understanding changes in glycan heterogeneity through the analysis of glycan synthesis and the regulation of glycosyl-transferases that synthesis both O- and N-type glycans.
- Horynová M, Takahashi K, Hall S, Renfrow MB, Novak J, Raška M.
Production of N-acetylgalactosaminyl-transferase 2 (GalNAc-T2) fused with secretory signal Igκ in insect cells.
Protein Expr Purif. 2012 Feb;81(2):175-80.
Support for this Work
NIH - NIGMS
- R01 GM098539-01 (Matthew Renfrow, University of Alabama at Birmingham, P.I.)Analytical Tools for the Analysis of Clustered O-glycans in Clinical Samples


