

 | |
 |
Home Lab Members Research Publications Collaborators Contact |
|
|
| ||||

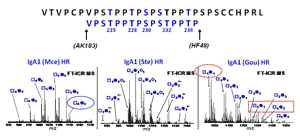
Our group makes use of high resolution FT-ICR MS to provide an accurate profile of entire population of O-glycosylated IgA1 proteins to identify the pathogenic forms contributing to the pathogenesis of IgA nephropathy. This includes the site-specific localization and characterization of individual O-glycan chains by use of electron capture/transfer dissociation (ECD & ETD). Our goal is to identify the aberrant IgA1 O-glycosylation pattern that leads to the mesangial deposits of IgA1-containing immune complexes. This may lead to alternative methods for diagnosing and monitoring the disease as well as identifying targets for therapeutic intervention.
 See Larger Image |
 See Larger Image |
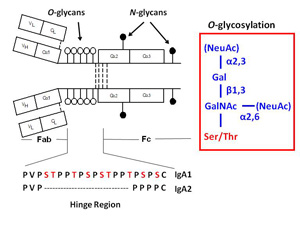
Glycosylation is one of the most common post-translational modifications of proteins. It is estimated that over half of mammalian proteins are glycosyalted. Several autoimmune disorders and chronic inflammatory diseases exhibit abnormal glycosylation of serum immunoglobulins. A variety of proteins are postranslationally modified with clustered sites of O-glycosylation. Serine and Threonine rich stretches within the amino acid sequence that have short O-glycan chains. Examples include the immunoglobulin A (A1 isotype), mucins, and bacterial cell surface proteins. For a given protein with sites of clustered O-glycans, the protein isolated from a single source is a population of variably O-glycosylated isoforms that usually show a distinct distribution of microheterogeneity in terms of number of chains, the sites of attachment and O-glycan composition at a given amino acid. Characterizing these clustered sites and understanding how the distributions change under differenct biological conditions or disease states is an analytical challenge.
IgA nephropathy (IgAN, also known as Berger's disease) is the most common primary glomerulonephritis worldwide, with about 20-40% of patients developing end-stage renal failure. It is characterized by mesangial deposits of IgA1-containing immune complexes. The carbohydrate side chains of IgA1 molecules play a pivotal role in the pathogenesis of IgAN. IgA1 contains a hinge region between the first and second heavy chain constant region domains with a high content of proline, serine, and threonine and usually have three to five O-linked glycan chains.
NIH- NIDDK