

 | |
 |
Home Lab Members Research Publications Collaborators Contact |
|
|
| ||||
 See Larger Image |
 See Larger Image |
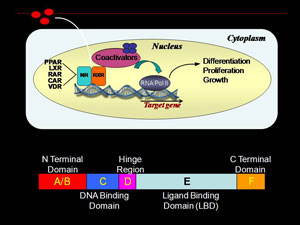
Retinoid X receptor (RXR) is a ligand dependent nuclear receptor transcription factor fundamental in regulation of cellular proliferation, differentiation and growth. Small molecule binding repositions key structural elements in RXR ligand binding domain (LBD), presenting/burying coregulator interaction surfaces. Agonist binding removes corepressor proteins and promotes interactions with transcriptional coactivators. RXR associated transcription complexes are of therapeutic interest for prevention and treatment of breast cancer. We are interested in the process of how ligand binding results in a conformational change in the ligand binding domain of RXR nuclear receptor transcription factor that leads to a specific transcriptional response. This ligand dependent process is often studied by use of X-ray crystallography. However, many of these resulting structures are very similar.
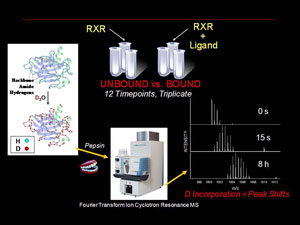
Our lab makes use of in-solution Hydrogen deuterium exchange (HD X) coupled with high resolution mass spectrometry (MS) to compare and contrast the changes in in-solution conformational dynamics of the RXR LBD when bound to different ligands and coactivators. Our current work centers around the UAB developed 9-cis-UAB30, an RXR specific retinoid that has shown great promise a chemopreventative agent. These HD X profiles provide a dynamic component to the structural analysis and can detect differences that ligand binding induces in the RXR LBD that are not observed in x-ray crystal analysis. These dynamic changes are quantative and can be used as structural markers to screen novel rexinoids for similar binding characteristics. We also correlate our results with more traditional molecular biology assays for characterizing novel RXR ligands in terms of ligand affinity and RXR activation. Overall, our goal is to provide a unique set of tools and perspective for understanding the process of ligand induced nuclear receptor activation.
This work has been supported by:
| © 2007 University of Alabama at Birmingham, Disclaimer UAB Department of Biochemistry and Molecular Genetics Website Design by Mikako Kawai and Matt Renfrow |