IgA Nephropathy
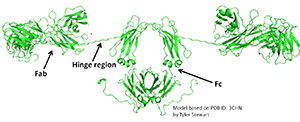
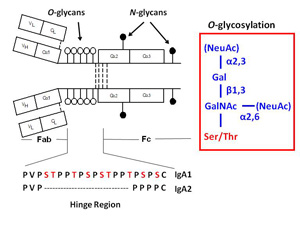
IgA nephropathy (IgAN, also known as Berger's disease) is the most common primary glomerulonephritis worldwide, with about 20-40% of patients developing end-stage renal failure. It is characterized by mesangial deposits of IgA1-containing immune complexes. The carbohydrate side chains of IgA1 molecules play a pivotal role in the pathogenesis of IgAN. IgA1 contains a hinge region between the first and second heavy chain constant region domains with a high content of proline, serine, and threonine and usually have three to six O-linked glycan chains.
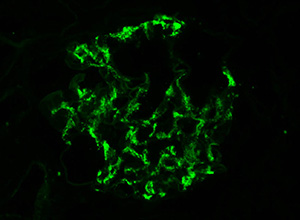
| ◄ Currently the only definitive diagnosis of IgAN is an IgA immunofluorescence stain of a renal biopsy. |
||
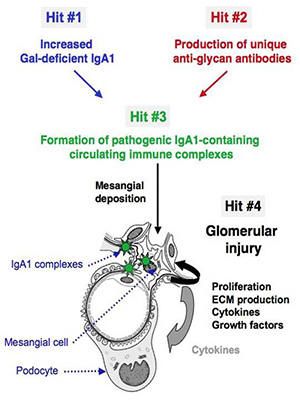
| ► Our propose multi-hit mechanism of IgAN pathogenesis (Suzuki et. al., 2011) |
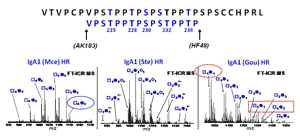
Analysis of clustered sites of O-glycosylation:
Our group makes use of high resolution FT-MS to provide an accurate profile of entire population of O-glycosylated IgA1 proteins to identify the pathogenic forms contributing to the pathogenesis of IgA nephropathy. This includes the site-specific localization and characterization of individual O-glycan chains by use of electron capture/transfer dissociation (ECD & ETD). Our goal is to identify the aberrant IgA1 O-glycosylation pattern that leads to the mesangial deposits of IgA1-containing immune complexes. This may lead to alternative methods for diagnosing and monitoring the disease as well as identifying targets for therapeutic intervention.
Clustered sites of O-glycosylation
Glycosylation is one of the most common post-translational modifications of proteins. It is estimated that over half of mammalian proteins are glycosyalted. Several autoimmune disorders and chronic inflammatory diseases exhibit abnormal glycosylation of serum immunoglobulins. A variety of proteins are postranslationally modified with clustered sites of O-glycosylation. Serine and Threonine rich stretches within the amino acid sequence that have short O-glycan chains. Examples include the immunoglobulin A (A1 isotype), mucins, and bacterial cell surface proteins. For a given protein with sites of clustered O-glycans, the protein isolated from a single source is a population of variably O-glycosylated isoforms that usually show a distinct distribution of microheterogeneity in terms of number of chains, the sites of attachment and O-glycan composition at a given amino acid. Characterizing these clustered sites and understanding how the distributions change under differenct biological conditions or disease states is an analytical challenge.
Iga Nephropathy
IgA nephropathy (IgAN, also known as Berger's disease) is the most common primary glomerulonephritis worldwide, with about 20-40% of patients developing end-stage renal failure. It is characterized by mesangial deposits of IgA1-containing immune complexes. The carbohydrate side chains of IgA1 molecules play a pivotal role in the pathogenesis of IgAN. IgA1 contains a hinge region between the first and second heavy chain constant region domains with a high content of proline, serine, and threonine and usually have three to five O-linked glycan chains.
Renfrow Lab Publications related to IgAN
-
Stuchlova Horynova M, Vrablikova A, Stewart TJ, Takahashi K, Czernekova L, Yamada K, Suzuki H, Julian BA, Renfrow MB, Novak J, Raska M.
N-acetylgalactosaminide α2,6-sialyltransferase II is a candidate enzyme for sialyation of galactose-defecient IgA1, the key autoantigen in IgA nephropathy.
Nephrol Dial Transplant. 2015 Feb;30(2):234-8.
- Takahashi K, Raska M, Stuchlova Horynova M, Hall SD, Poulsen K, Kilian M, Hiki Y, Yuzawa Y, Moldoveanu Z, Julian BA, Renfrow MB, Novak J.
Enzymatic sialylation of IgA1 O-glycans: implications for studies of IgA nephropathy.
PLoS One. 2014 Jun 11;9(2):e99026.
- Hastings MC, Moldoveanu Z, Suzuki H, Berthoux F, Julian BA, Sanders JT, Renfrow MB, Novak J, Wyatt RJ.
Biomarkers in IgA nephropathy: relationship to pathogenetic hits.
Expert Opin Med Diagn. 2013 Nov;7(6):615-27.
- Mestecky J, Raska M, Julian BA, Gharavi AG, Renfrow MB, Moldoveanu Z, Novak L, Matousovic K, Novak J.
IgA nephropathy: molecular mechanisms of the disease.
Annu Rev Pathol. 2013 Jan 24;8:217-40.
- Novak J, Renfrow MB, Gharavi AG, Julian BA.
Pathogenesis of immunoglobulin A nephropathy.
Curr Opin Nephrol Hypertens. 2013 Mar 18. [Epub ahead of print]
- Franc V, Rehulka P, Raus M, Stulík J, Novak J, Renfrow MB, Sebela M.
Elucidating heterogeneity of IgA1 hinge-region O-glycosylation by use of MALDI-TOF/TOF mass spectrometry: Role of cysteine alkylation during sample processing.
J Proteomics. 2013 Jul 24. [Epub ahead of print]
- Takahashi K, Smith AD, Poulsen K, Kilian M, Julian BA, Mestecky J, Novak J, Renfrow MB.
Naturally occurring structural isomers in serum IgA1 o-glycosylation.
J Proteome Res. 2012 Feb 3;11(2):692-702.
- Novak J, Julian BA, Mestecky J, Renfrow MB.
Glycosylation of IgA1 and pathogenesis of IgA nephropathy.
Semin Immunopathol. 2012 May;34(3):365-82.
- Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA.
The pathophysiology of IgA nephropathy.
J Am Soc Nephrol. 2011 Oct;22(10):1795-803.
- Takahashi, K., Wall, S.B., Suzuki, H., Hall, S. Smith, A.D.,IV, Poulsen, K., Kilian, M., Julian, B.A., Mestecky, J.,
Novak, J., Renfrow, M.B.,
"Clustered O-glycans of IgA1: Defining macro- and micro- heterogeneity by use of electron capture/transfer dissociation"
Mol. Cell. Proteomics Epub Sep 7. 2010
- Wada, Y., Dell, A., Haslam, S.M., Tissot, B., Canis, K., Azadi, P., Backstrom, M., Costello, C.E., Hansson, G.C., Hiki, Y., ishihara, M., Ito, H., Kakehi, K., Karlsson, N., Koichi, K., Kawasaki, N., Khoo, K.-H., Kobayashi, K., Kolarich, D., Kondo, A., Lebrilla, C., Nakano, M., narimatsu, H., Novak, J., Novotny, M.V., Packer, N.H., Renfrow, M.B., Tajiri, M., Thomsson, K.A., Yu, S.-Y., Taniguchi, N.
Comparison of methods for profiling O-glycosylation: HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1.
Mol Cell. Proteomics. Apr 9(4):719-27, 2010
- Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB.
Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding.
Biochemistry. 28;47(43):11285-99, 2008
- Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J.
Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res. 31:29-37, 2008
- Novak, J., Moldoveanu, Z., Renfrow, M.B, Yanagihara, T., Suzuki, H., Raska, M., Hall, S., Brown, R., Huang, W.-Q., Goepfert, A., Kilian, M., Poulsen, K., Tomana, M., Wyatt, R.J., Julian, B.A., Mestecky, J.
IgA Nephropathy and Henoch-Schoenlein Purpura Nephritis: Aberrant Glycosylation of IgA1, Formation of IgA1-Containing Immune Complexes, and Activation of Mesangial Cells.
Contrib Nephrol. 157:134-8, 2007
- Renfrow, M.B., Mackay, C.L., Chalmers, M.J., Julian, B.A., Mestecky, J., Kilian, M., Poulsen, K., Emmett, M.R., Marshall, A.G., Novak, J.
Analysis of O-glycan Heterogeneity in IgA1 Myeloma Proteins by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry: Implications for IgA Nephropathy.
Anal. Bioanal. Chem., 389:1397-407, 2007
- Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J.
Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry.
J Biol Chem. 280:19136-19145, 2005.
UAB IgA Nephropathy Group Publications
More information on IgA Nephropathy
- NIH/NIDDK
- IgA Nephropathy Foundation of America, Inc
- Reviews on IgA Nephropathy research
- Support IgA Nephropathy Research at UAB
(Select – “See all funds” followed by “School of Medicine” followed by “IGA Nephropathy Research” )
Basic descriptions of IgA Nephropathy
Support for this work
NIH – NIGMS
- R01 GM098539-01 (Matthew Renfrow, University of Alabama at Birmingham, P.I.) Analytical Tools for the Analysis of Clustered O-glycans in Clinical Samples
NIH – NIDDK
- R21 DK077279-01A2 (Matthew Renfrow, University of Alabama at Birmingham, P.I.)
Accurate profiles of IgA1 O-glycan Heterogeneity in IgA Nephropathy - R01 DK 71802-01A1 (Andy Herr, University of Cincinnati, P.I.)
IgA1 Glycosylation and Receptor Interactions in IgA Nephropathy - R01 DK78244-01 (Jan Novak, University of Alabama at Birmingham P.I.)
Molecular Basis of Pathogenicity of IgA1-containing Immune Complexes - R21 DK080301-02 (Jan Novak, University of Alabama at Birmingham, P.I.)
IgA-secreting B cell lines: a novel tool for studies of IgA nephropathy - R21 DK75868 (Jan Novak, University of Alabama at Birmingham, P.I.)
Urinary Polypeptide Biomarkers of IgA Nephropathy