Media contact: Beena Thannickal
 It sounds like something out of a sci-fi movie: Researchers take ordinary soldiers, train each one to seek and destroy a single enemy, then replicate them millions of times over to create an unstoppable clone army. That’s one way to visualize what happens when scientists make monoclonal antibodies. These powerful medical weapons don’t get much attention outside the research community, but they’re an indispensable part of the new era of precision medicine. They’ve already revolutionized treatment of cancer and autoimmune diseases. Zika, Ebola and HIV may be next. And throughout their long and fascinating history, from the 1970s to the present, UAB researchers have played a leading role.
It sounds like something out of a sci-fi movie: Researchers take ordinary soldiers, train each one to seek and destroy a single enemy, then replicate them millions of times over to create an unstoppable clone army. That’s one way to visualize what happens when scientists make monoclonal antibodies. These powerful medical weapons don’t get much attention outside the research community, but they’re an indispensable part of the new era of precision medicine. They’ve already revolutionized treatment of cancer and autoimmune diseases. Zika, Ebola and HIV may be next. And throughout their long and fascinating history, from the 1970s to the present, UAB researchers have played a leading role.
The human immune system is usually very good at detecting and eliminating invaders. The vast majority of the time, it has no trouble fighting off bacteria, viruses, worms and more. But sometimes, in the case of many cancers, or HIV or autoimmune diseases such as rheumatoid arthritis, it fails. The immune system gets thrown off the scent (cancer) or overwhelmed (HIV). Or it gets confused and starts attacking healthy cells (rheumatoid arthritis). But what if scientists could intervene just enough to fix the problems, freeing the immune system to fight malicious cells?
That dream, spurred by Paul Ehrlich's work on antibodies in the 1900s, finally seemed truly viable 70 years later. In 1975, Drs. Georges Kohler and César Milstein demonstrated their breakthrough “hybridoma” technique. By fusing myeloma cancer cells with antibody-producing B cells, Kohler and Milstein created an immortal source of cells that could home in on a single target in the body: monoclonal antibodies, or Mabs. Kohler and Milstein, along with Niels Jerne, Ph.D., won the Nobel Prize for this work in 1984. Two years later, the first FDA-approved Mab reached the market. The drug, Orthoclone, blocks an activating receptor on the immune system’s T cells, helping to prevent kidney transplant rejection.
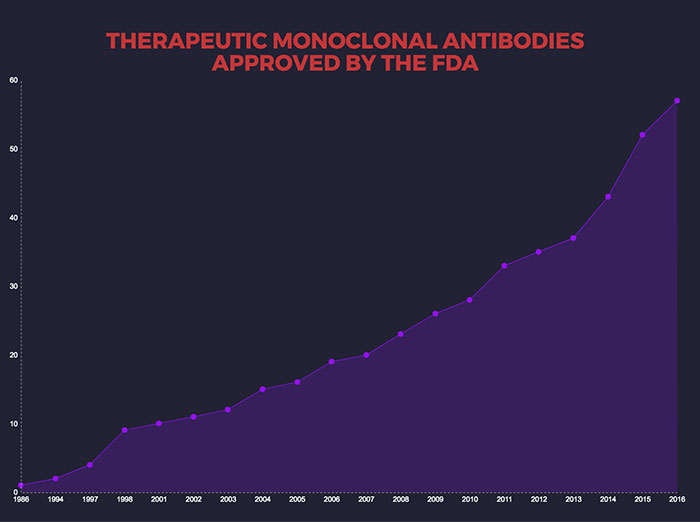
There are now dozens of Mabs available to treat a wide range of conditions, including cancer, rheumatoid arthritis, multiple sclerosis, cardiovascular disease, lupus, psoriasis and transplant rejection. One of the most exciting new classes of anti-cancer drugs, the checkpoint inhibitors — credited with saving Jimmy Carter’s life — are monoclonal antibodies. A November 2016 research report estimated the worldwide Mab market at $85.4 billion, and predicted it would rise to $138.6 billion by 2024, driven by "increasing demand for personalized medicine."
The bridge between Kohler and Milstein’s 1975 breakthrough and today’s Mab therapy revolution was a tremendous amount of research, in the lab and in the clinic. And a great deal of that work was done by pioneering scientists at UAB. Here, several key figures share their recollections of those groundbreaking days.
 John Kearney in his UAB lab. Photo courtesy UAB Archives.
John Kearney in his UAB lab. Photo courtesy UAB Archives.
Waiting for the miracle
In 1978, John F. Kearney, Ph.D., was a newly minted faculty member in the UAB Department of Microbiology. A mentor suggested he be sent off on sabbatical to “get new ideas.” John Durant, M.D., founding director of UAB’s Comprehensive Cancer Center, contributed funds, and Kearney and his family headed to Germany for a temporary appointment in the lab of Klaus Rajewsky, M.D., at the University of Cologne.
It was an exciting time. Kohler and Milstein’s work had demonstrated that monoclonal antibodies had great potential both as research tools and as clinical treatments. But their hybridoma technique suffered from a serious flaw: it didn’t produce “pure” Mabs. The process involved injecting a target antigen (such as a tumor sample) into an animal model and harvesting the B cells that produced the desired antibodies. Then that B cell could be fused with an immortal plasma tumor cell (the "fusion partner") to create a new cell, the hybridoma, which could churn out a nearly unlimited quantity of that one specific antibody. In practice, however, the fusion partner also created mixed-chain antibodies, which contaminated the desired end product.
John Kearney: “I was fortunate to stumble onto this project. Klaus's lab had jumped on the hybridoma technology real fast because he was quick to realize the power of the technology. He was friends with Cesar Milstein and had acquired cell lines from him. However, the fusion partners developed at the time still had their own immunoglobulin [IG] chains, and so they would make mixed molecules — you would make some antibodies that weren’t specific, and some that were. Klaus had realized that we needed to make a fusion partner that didn't have any IG chains of its own.
“They were using flow cytometry to look for antibody-negative fusion partners and it hadn't worked. So what I did — and people thought this was foolhardy — was I took one of the cell lines they made and grew up multiple clones from single cells of the mother cell line, made smears of hundreds of these clones, stained them and checked to see if any had lost the ability to make IG chains.
"Nobody else would have done it that way because it was so tedious and unlikely to succeed. I had to borrow an instrument from another group in Cologne to make the smears because Klaus's lab did not have one. It was back-breaking work, but I kept at it out of sheer stubbornness. I did about 600. And eventually to our delight we found one that had lost immunoglobulin secretion."
| "It was back-breaking work, but I kept at it out of sheer stubbornness. I did about 600." |
That cell line, known as P3X63Ag8.653, "became famous worldwide instantaneously," recalled Rajewsky. Kearney's paper describing the work, “A New Mouse Myeloma Cell Line that Has Lost Immunoglobulin Expression but Permits the Construction of Antibody-Secreting Cell Lines,” was published in the Journal of Immunology in 1979. "This clone," Kearney wrote, "can be used for efficient fusion with antibody-forming cells to obtain hybrid cell lines producing pure monoclonal antibodies."
Kearney: "I came back to UAB with the cell line. I really liked making these things, so I helped a lot of people around here make antibodies and taught them how to make them. As a rule, we would make them on a Friday afternoon. We would immunize the animals for a few weeks beforehand, but the actual process after that only takes a few hours. We would get it ready, then go off to The Burly Earl [a local pub] and wait for the miracle to come.
"We also set up a hybridoma core facility, which was run first by Denise Shaw, and then for many years by Mary Ann Accavitti-Loper. There are new technologies now — phage display and libraries of immunoglobulin genes, for example — that are starting to supersede monoclonal production, but they'll never replace it totally. You still can't beat the natural immune system. The way it makes these antibodies is fantastic, all the selection that goes on in the lymphoid tissues."
Flavius Martin, M.D., joined Kearney’s lab in the early 1990s as a postdoctoral researcher. He is now at biotechnology giant Amgen, where he leads discovery research for inflammation and oncology, including Mab program development.
Flavius Martin: "John generated this novel fusion partner that made the process of generating hybridomas very efficient. It became so useful and easy to use that it became widespread in the academic community, and the individuals that started looking into this for therapeutic purposes, most of that community started using this cell line that John had made. Over a few years that technology evolved, but in many ways the work John did in Germany really helped the field explode.
"When he came back to the United States, he started applying these monoclonal antibodies to a lot of important biological questions that were really at the frontiers of the field — using monoclonal antibodies to characterize what is really going on in the immune system."
 Al LoBuglio and M.B. Khazaeli. Photo courtesy UAB Archives.
Al LoBuglio and M.B. Khazaeli. Photo courtesy UAB Archives.
Seeking the magic bullet
As Kearney and other basic scientists were developing Mab technology in the late 1970s and early 1980s in their labs, clinicians were searching for ways to bring Mabs to patients.
Albert LoBuglio, M.D., who would become the UAB Cancer Center’s second director in 1983, was then director of the Division of Hematology and Oncology and the Simpson Memorial Research Center at the University of Michigan Medical Center. LoBuglio had become interested in antibodies as a young researcher in Boston, publishing a number of important observations of how antibody molecules mediate disease.
Albert LoBuglio: “In the early 1980s, a number of institutions had figured out how to use monoclonal antibodies in research. When I arrived at the University of Michigan there was a young basic scientist there in the pathology department named M.B. Khazaeli, who was making monoclonal antibodies for laboratory testing. We met and started to do joint research. Since I had all this antibody background it was sort of a natural transition to monoclonals. We used them to produce novel laboratory tests and animal models of cancer."
LoBuglio’s excitement about Mabs caught the attention of Mansoor Saleh, M.D., a young resident at the Henry Ford Hospital in Detroit.
Mansoor Saleh: "Dr. LoBuglio came to Henry Ford to give Grand Rounds, and he used the term 'magic bullet' — a targeted antibody therapy that would only kill cancer cells. I thought that was exactly what I wanted to do, so I applied to the fellowship program at Michigan and was accepted. Then I found out he had taken the job at UAB, and I said, 'Ok, I'll go to Alabama instead.'"
LoBuglio: "I got interested in UAB because it was clear that the interaction of faculty across departments wasn't just something that was talked about, but something that built the institution and moved it forward. When we came here, it seemed like an ideal time to put a team together specifically for monoclonal antibody studies."
 Mansoor Saleh. Photo courtesy UAB Archives.
Mansoor Saleh. Photo courtesy UAB Archives.
Saleh: "At that time, Dr. LoBuglio was working on idiopathic thrombocytopenic purpura, or ITP, looking at antibodies that target the platelets, which results in those platelets being destroyed by the immune system. We published a number of papers. Our group at UAB was one of the first to qualitate the amount of antibody on the platelet surface. Very quickly thereafter, the question became, Can we make antibodies against cancer?"
Andres Forero, M.D., trained as a fellow with LoBuglio in the 1980s, and has spent much of his career at UAB. He is now a senior scientist and director of the Breast Cancer Program at the UAB Comprehensive Cancer Center.
Andres Forero: "Dr. LoBuglio was one of the first people who saw the future of monoclonal antibodies. Thirty years ago, there were two ways to go in cutting-edge cancer research: one was vaccines, and the other was Mabs. One of the nice things about Dr. LoBuglio is that he was interested in both and worked in both areas. Vaccines, unfortunately, have failed dramatically. Not a single one is available today. But the Mab pathway has become one of the most successful stories in the treatment of cancer."
| "The Mab pathway has become one of the most successful stories in the treatment of cancer." |
First in humans
LoBuglio: "We were in the vanguard looking at use of monoclonals in humans. There had been no mouse monoclonal studies in humans, except small, individual studies in Europe. Our group did the first mouse monoclonal antibody study in the United States with a formal protocol. There had been considerable expertise at UAB in basic immunology. We added a radioisotope labeling person, and a physician to lead the trials and lab people who could do correlative studies."
Saleh: "There was a company that made antibodies against a target that seemed to be prevalent on tumor cells. They were not tumor-specific, because those targets were also on normal cells, but we had to at least give it a try. We were able to show that, yes, a patient can receive a mouse Mab targeting the tumor. But the half-life of the mouse antibody was very short — a few hours.
"We could give one dose with no problem. By the second dose, the patient had already made an immune response to the mouse, so by the time you gave the third dose it was destroyed very quickly. You really couldn't use this for therapeutic purposes, although you could use it for imaging."
LoBuglio: "Our paper said that mouse monoclonal antibodies were impractical, and defined regions that produced the immune response and said that was the area we had to get rid of. So in collaboration with a company called Centocor, we developed a genetically engineered monoclonal antibody that was part mouse and part human."
Saleh: "We were using recombinant DNA technology. We knew the gene for the Fc portion of the antibody [which interacts with a patient’s immune system], so we took the gene for the human Fc portion, and the gene for the mouse Fab portion, which binds to the target. It was 80 percent human and 20 percent mouse: that's a chimeric antibody.
"The kinetics for the chimeric antibody was multiple days. It wasn't 21 days, which is the half-life of human immunoglobulin, but it was much longer than mouse antibodies. About 5-7 days, as opposed to 5-6 hours."
LoBuglio: "We did the first trial in a human and it worked well. Our conclusion was that these genetically engineered antibodies were what had to be used for efficacy."
| "We did the first trial in a human and it worked well." |
Subsequent studies at UAB looked for therapeutic effects, but the response was poor.
LoBuglio: "We pointed out that the antibodies probably needed to interact with a receptor or molecule that was vital to the survival of the tumor. It couldn't just attach — it had to be something that was critical. You had to find molecules on the surface of the tumor cells that could be inhibited to damage the tumor. It was clear that lots of monoclonal antibodies could localize to tumor sites, and if you radiolabeled them, which we did, you could tell it was getting there. It just wasn't getting to a deficit of the tumor. We had to find a vital molecule on the surface of the cell.
"Rituxan was one of the drugs that had that benefit. It reacted with CD20 [a cell membrane protein of the tetraspanin family]. Just the attachment interfered with cancer cells and killed them in the test tubes."
Rituxan was developed by a company called IDEC Pharmaceuticals, based in San Diego, California. LoBuglio and Kearney both had advisory roles with IDEC.
Kearney: "Sometimes Al LoBuglio and I would fly over to the meetings together. He would go to the clinical meetings, and I would go to the basic science meetings. One day the executives and scientific managers presented their plan to make an antigen specific to CD20, which is present on all B cells. We experts thought this was a crazy idea; CD20 is so widespread, we thought it just wouldn't be effective. It would be absorbed away. But they went ahead against the conventional wisdom. And that drug, Rituxan, the first to be licensed by the FDA, became extremely successful for treatment of certain B cell tumors and now many other human diseases involving B cells."
LoBuglio: "We had quite a reputation by then. We ended up with faculty in pathology, radiation therapy, immunology, internal medicine. The kinds of work that are usually done in the mouse we were able to do in humans. The major reason for getting all these trials was the expertise that we had in monoclonals and design of experiments. Within three or four years, we could select whatever partners we wanted from the industrial side of the monoclonal antibody field. It's a $20 million or $30 million investment to make enough Mabs to give to humans, so companies are looking for people who can do this well."
| "It's a $20 million or $30 million investment to make enough Mabs to give to humans, so companies are looking for people who can do this well." |
"One company working with us made a genetically engineered antibody directed at an epidermal growth factor receptor. That trial showed that antibodies plus radiation could shrink tumors. We published that and then went in and did pivotal trials for FDA approval of the drug, which became Erbitux. That's still one of the primary treatments for head and neck cancer, and the pivotal trial was done here with our chair of radiation therapy as the principal investigator.
"We also did a whole series of antibody trials with toxins attached to the antibody so that it delivered a drug or toxic molecule."
Saleh: "It's like a trojan horse. You have antibodies linked to chemotherapy, so the antibody attaches to the tumor and the chemotherapy sneaks in. You have to have a linker between the antibody and the chemotherapy payload, and the linkers had always been sort of shaky. Our first paper with that involved an immunoconjugate where the antibody was very potent but the linker was very shaky, and the chemotherapy would come off. There was quite a bit of toxicity. We were the first one to do an immunoconjugate therapy in humans here at UAB, but it was ineffective. We showed the immune response, but also that the linker was weak and the chemotherapy was coming off."
LoBuglio: "In a group of patients with Hodgkin’s disease we showed that there were benefits. Several years later we took part in pivotal trials of that drug, known as Adcetris, and it's used today."
 Andres Forero discusses a UAB clinical trial with a care team member at the Kirklin Clinic.
Andres Forero discusses a UAB clinical trial with a care team member at the Kirklin Clinic.
Forero: "The first person treated in the first clinical trial of Adcetris was one of my patients here at UAB. We went through all the steps of those studies, and it was approved by the FDA for Hodgkin's lymphoma a few years ago. It has dramatically improved treatment."
LoBuglio: "In fact, we helped start the company that put that molecule together. The head of immunology at one of the major drug companies had decided to shut down its immunology section. We had been doing a lot of collaborations with them, so one of their researchers came and visited us and said he was going to start his own company and negotiate ownership of the antibody's structure. We helped write the business plan for that company, which is now called Seattle Genetics. They are a terrific company with a whole lot of antibody-drug conjugates now being studied."
Saleh: "A number of the pharmaceutical connections that we developed as a result of Dr. LoBuglio's reputation at that time are the same ones that we work with today in the UAB Phase 1 Clinical Trials Program." [The program, launched in 2015, is directed by Dr. Saleh.] Right now, we've got 20 trials, and two-thirds of those include the delivery of monoclonal antibodies against specific targets."
 Flavius Martin. Image courtesy Amgen.
Flavius Martin. Image courtesy Amgen.
On the frontiers
The excitement surrounding monoclonal antibodies is still infectious. Flavius Martin, who was a postdoctoral researcher in John Kearney's lab from 1993 to 2002, is now vice president for oncology and inflammation discovery research at Amgen, which is the world's largest independent biotech company.
Martin: "I'm originally from Romania. I went to medical school there, and during my training it became clear that my passion was in biomedical research. I started going to professional meetings, and I met John. The next year, I went to see him and Max Cooper at the International Immunology Meeting in Budapest. This must have been 1991 or thereabouts. Through those interactions it became clear to me what a spectacular place UAB was, and I started getting more familiar with what John was about in terms of science and his contributions to the early days of monoclonal antibody characterization. I was very fortunate that he selected me and allowed me to come to UAB to learn.
"John and I did a lot of work using cutting-edge genetic systems as well as monoclonal antibody technology that John had developed. We were quite successful at making a mark and moving the field forward in understanding the basic properties of B cell function and how that translates into various aspects of immune system function.
"Eventually, I realized that I wanted to contribute in drug development and making medicines against human disease. I chose to join the department of immunology at Genentech, in south San Francisco, where I started applying my knowledge of monoclonal antibodies. There is an enormous amount of science that goes into using these molecules as therapeutics, which started with the earliest discoveries of Kohler and Milstein. They were making Mabs on a scale that was sufficient for academic research. To take that and make an industrial process out of it, a big scientific infrastructure has to be built. The process that people used to do in a 20-milliliter flask has been scaled up and now is done in reactors that hold 10,000 liters or more. These are enormous production yields, and you can't have any down time.
"What is amazing is how well evolution produced the antibody molecule and allowed it to be very versatile. You can introduce a lot of change — take pieces of this molecule, make them bigger, smaller, make changes to the structure that allow you to increase or decrease the properties that you do or don't want.
| "We will eventually reach the limit of what is achievable, but we haven't reached that yet." |
"One way to take advantage of these wonderful molecules is to combine properties of two monoclonal antibodies. These are now starting to be used across industry quite a bit. Maybe one of the most famous and interesting examples is in immuno-oncology, which uses activation of a patient's immune system to clear out a tumor. One can take parts from one monoclonal antibody against the tumor and another against a T lymphocyte, which is really the premier cell that can kill a tumor cell, put them together through molecular engineering and use that as a therapeutic. The tumor cells are coated with molecules that allow them to be recognized efficiently by T lymphocytes. The first such molecule that reached the market, known as Blincyto, is used in patients with acute lymphoblastic leukemia.
"The frontier is making these multifunctional molecules that have more than one target. The field is looking toward bifunctional and even trifunctional molecules. The challenge is preserving the functionality and thinking that in the end this needs to be a drug that is viable to manufacture and scale up. The more we change them, the more complex the manufacturing process will get. We will eventually reach the limit of what is achievable, but we haven't reached that yet."
 John Kearney's lab continues to produce groundbreaking science. In 2016, Kearney, graduate student Preeyam Patel and microbiology faculty member R. Glenn King reported on an experimental protective therapy for asthma.
John Kearney's lab continues to produce groundbreaking science. In 2016, Kearney, graduate student Preeyam Patel and microbiology faculty member R. Glenn King reported on an experimental protective therapy for asthma.
An enduring legacy
In 2016, Kearney received a career award from the American Association of Immunologists, the AAI-BioLegend Herzenberg Award, for "outstanding research contributions to the field of immunology in the area of B cell biology." His research has uncovered several fundamental facets of B cell biology and had applications from juvenile diabetes to biological warfare. The Kearney lab currently is exploring new therapies for asthma and allergies, among other projects.
Kearney: "As I look back, 90 percent of what I've done can be traced back to the cell line I developed at the University of Cologne. UAB also licensed a lot of the hybridomas we created, and they still bring in quite a bit for the university, hundreds of thousands of dollars per year.
“Multiple groups around the world are working on Mabs for Ebola, Zika, HIV — there’s just incredible potential. At the same time, there are fundamental questions about Mabs that still need to be resolved, especially about the targets. That’s why you have to have basic research."
Forero: "UAB has been the first in humans in 20 to 30 therapies over the past five years. We work with both large firms and small biotechs that have started to develop exciting new drugs. That allows our patients to have access to these treatments earlier. Adcetris, for example, can cure cases of Hodgkin's lymphoma, and for years, the only way for people in Alabama to get this drug was at UAB. Our research has brought many benefits to the citizens of this state."