UAB Researchers Focus On New Clues to Macular Degeneration
By Bob Shepard
 |
Christine Curcio has studied age-related eye diseases throughout her 28-year career as an eye researcher. |
Oversized phone dials. Magnifiers. Little tricks with peripheral vision. There are many ways to cope with age-related macular degeneration (ARMD). Unfortunately, one of the most popular is denial.
“Half of my new patients at the UAB Center for Low Vision Rehabilitation want me to make them a new pair of glasses so they can go home and have everything the way it was before,” says UAB optometrist Dawn DeCarlo, O.D., the center’s director. “Many patients have no idea what ARMD really means.”
For a long time, even researchers didn’t know a great deal about this devastating condition. And even though they’ve made progress in recent years, they still face some maddening blind spots. The disease, as its name implies, is associated with aging, and it gradually destroys sharp, central vision. ARMD comes in two forms: wet and dry. The wet form, which affects about 15 percent of those with ARMD, leads to more severe vision loss, but its cause is reasonably well understood. In a process called neovascularization, abnormal blood vessels behind the retina grow into the macula, the center of the retina. These blood vessels can rupture and leak, damaging the macula by separating it from the rest of the retina. The good news is that there are new medications, called anti-VEGF drugs, that are extremely effective in treating the wet form of the disease.
Dry Discoveries
The dry form, which affects many more people and can be a precursor to wet ARMD, is not well understood at all. But scientists are beginning to discover some important clues. “There are at least three processes going on” simultaneously before wet ARMD, says UAB eye scientist Christine Curcio, Ph.D., one of the world’s leading ARMD researchers. “There is death of the photoreceptors at the back of the retina, death of the retinal pigment epithelium (RPE) cells behind the photoreceptors, and accumulation of lesions along a wall called Bruch’s membrane, behind the RPE layer.”
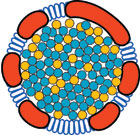 |
A lipoprotein particle from Bruch's membrane, an eye region at the back of the retina. UAB's Christine Curcio thinks the buildup of these particles—similar to the buildup of cholesterol in coronary arteries—plays a major role in the development of age-related macular degeneration. Illustration by David Fisher, UAB Medical Education and Design Services. |
Curcio has been studying age-related eye diseases throughout her 28-year career as an eye researcher. Her current focus is on Bruch’s membrane and the RPE cell layer. In fact, she’s intrigued by a tiny area, only one to two micrometers wide, that serves as the boundary between RPE and Bruch’s membrane. Curcio thinks a buildup of lipoprotein particles in that boundary—similar to the buildup of cholesterol in coronary arteries—plays a major role in the development and progression of ARMD.
Curcio’s lab has discovered that RPE cells are capable of taking up LDL (low-density lipoprotein) and HDL (high-density lipoprotein)—the bad and good cholesterol that are so important in heart disease—from the bloodstream. It turns out that these lipoproteins deliver nutrients needed by the photoreceptors and the retina—nutrients such as lutein and vitamin E—to the RPE.
“Our current hypothesis is that there is a kind of recycling mechanism taking place in the RPE,” says Curcio. “Plasma lipoproteins are taken in, they are stripped of the nutrients the retina needs, and then they are repackaged and sent back out again.”
Decades of Detritus
So what does this have to do with ARMD? In research models of the disease, Curcio has found age-related accumulations of repackaged lipoproteins piling up in Bruch’s membrane.
"All older people have accumulated particles in Bruch’s membrane—some have more of this stuff than others—but until we started studying this process, nobody knew that this accumulation was taking place,” she says.
Curcio believes the normal scattering of lipoproteins in Bruch’s membrane probably does not cause any vision problems. But in some people, these lipoproteins seem to clump together and settle at the boundary between Bruch’s and the RPE cells. These clustered lipoproteins form a barrier described as a “lipid wall” by a postdoctoral fellow working with one of Curcio’s collaborators.
“Layer of Goo”
The lipid wall prevents water from passing into and out of the RPE cells, which creates a problem because water is essential for transporting important components between cells. The lipid wall also destabilizes the structural integrity of the RPE cell layer, Curcio says.
“Imagine the lipid wall as sort of a layer of oil droplets,” she explains. “The RPE is floating on this layer of goo and is no longer solidly attached to the Bruch’s membrane, leaving the RPE cut off from nutrients it needs to survive.”
This same boundary area is also the location where extracellular debris called drusen form. Drusen are indicators of advancing ARMD, bolstering Curcio’s concept.
“People argue over whether drusen are bad for you,” she says. “I think drusen are a marker for a larger problem, because they are indicative of poor RPE health. The end stages of ARMD are defined as the loss of the RPE layer. If the RPE is in poor health or is gone entirely, the photoreceptors will go, too. And loss of photoreceptors means you can’t see.”
Potential Interventions
If Curcio is right about the lipid wall, her findings will give science a fresh target for new imaging and diagnostic techniques, as well as another point of intervention for drug therapy—although she cautions that there is much to be learned about the physiology of the back of the eye before drug trials can begin.
 |
Dawn DeCarlo, director of the UAB Center for Low Vision Rehabilitation, helps educate patients on the effects of age-related macular degeneration. |
But if lipoproteins are part of the ARMD puzzle, then eye researchers may already have a powerful pharmaceutical ally at their disposal in the form of anti-cholesterol drugs. Cardiologists have been studying lipoproteins for years in their efforts to treat high cholesterol and atherosclerosis.
“One good thing for scientists, physicians, and ARMD patients is that a lot of the research into cholesterol and managing LDL has already been done in the field of heart disease,” Curcio says. “We don’t have to start from scratch. We can build off the outstanding work that has already been done and paid for.”
And it won’t take a complete cure to make a world of difference to patients in clinics such as UAB’s Center for Low Vision Rehabilitation. According to Dawn DeCarlo, any advances that help slow down ARMD or speed up diagnosis are helpful. “Patients with earlier stages of ARMD have more rehabilitation options that can substantially improve their visual abilities,” she says. “The anti-VEGF drugs have made a world of difference. They don’t cure the disease, but they often maintain it in a state that is much more amenable to rehabilitation. Hopefully, with the work of scientists like Dr. Curcio, we’ll have treatments for dry ARMD that have the same effect on rehabilitation and ultimately on quality of life.
“I see people with ARMD succeed all the time,” DeCarlo adds. “If we can treat the degeneration, slow it down by even a couple of years, and provide them with the training and tools to cope with the changes in their vision, then we’ve done something and our patients are grateful. I got three hugs yesterday. You know it’s a good day when you get that many hugs.”
More Information
UAB Department of Ophthalmology