Discovery Reshapes Textbooks and TB Treatment
By Chris Jones
 Discoveries in the lab of Michael Niederweis have helped reshape scientists' understanding of the bacterium that causes tuberculosis.Michael Niederweis, Ph.D., has spent most of his career trying to breach two formidable barriers. The first is the cell wall of Mycobacterium tuberculosis, the bacterium that causes tuberculosis (TB), which causes more deaths each year than any other bacterial pathogen. The second is the opposition of the TB research community, which has been slow to accept the UAB microbiologist’s revolutionary discovery about that wall.
Discoveries in the lab of Michael Niederweis have helped reshape scientists' understanding of the bacterium that causes tuberculosis.Michael Niederweis, Ph.D., has spent most of his career trying to breach two formidable barriers. The first is the cell wall of Mycobacterium tuberculosis, the bacterium that causes tuberculosis (TB), which causes more deaths each year than any other bacterial pathogen. The second is the opposition of the TB research community, which has been slow to accept the UAB microbiologist’s revolutionary discovery about that wall.
Bacteria have evolved two types of cell walls to protect themselves from their environments. Some have a slimy, sugary coat while others have an outer membrane, like the wall of a fortress. TB researchers had long believed that the Mycobacterium tuberculosis bacterium had a cell wall that was unique but nevertheless similar to the sugar-coated bacteria. But Niederweis and his team have shown evidence for an outer membrane, a finding that could have a profound impact on the development of new TB drugs.
|
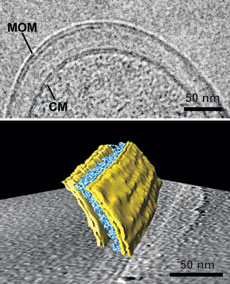
The mycobacterial outer membrane (MOM) from Mycobacterium bovis BCG is visualized by cryosectioning (top) and by cryo-electron tomography (bottom). The periplasmic space between the MOM and the cytoplasmic membrane (CM) contains the layers of the arabinogalactan-peptidoglycan polymer (indicated in blue in the 3-D representation). For further information see Proc. Natl. Acad. Sci. U S A 105: 3963-3967 (2008).
Left: Side view. Right: top view. Alternating adjacent monomers are colored in red and blue. For further information see Science 303: 1189-1192 (2004).
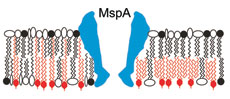
Mycolic acids are drawn in red and inserted either in the elongated conformation (model I, left side) or the folded conformation (model II, right side). A porin, here MspA from M. smegmatis, is represented in blue. The symbols of lipid head groups indicate that different free lipids may occur in both leaflets of the outer membrane. For further information see Trends Microbiol 18: 109-116 (2010). |
However, it hasn’t been easy convincing people that the textbooks are wrong. Many of the papers that Niederweis and his colleagues submitted to journals were met with skepticism, he says. “Peer reviewers would say our work is innovative, and they liked how we did it as a lab, but they thought, ‘Well, he is just a crazy guy who works on something that doesn’t exist,’” he recalls.
Solving the Membrane Mystery
Niederweis found the first clue to the existence of an outer membrane in a 1992 report demonstrating that the cell wall of a bacterium related to Mycobacterium tuberculosis might contain water-filled channel proteins called porins. These porins act like doors in a wall, allowing for small molecules to pass through outer membranes without compromising the integrity of the cell, he explains.
“The existence of these porins would make sense only if mycobacteria have an outer membrane,” he says. Niederweis and his colleagues isolated and characterized a mycobacterial porin, and their findings, published in the journal Science in 2004, strengthened the idea that Mycobacterium tuberculosis might have an outer membrane.
However, the TB field remained skeptical. Niederweis’s team then used cyro-electron microscopy to visualize the cell wall in its natural state. In 2008, working with a group at the Max Planck Institute for Biochemistry in Germany, Niederweis and colleagues published images of the outer membranes of bacteria similar to Mycobacterium tuberculosis. Since then, other scientific groups have independently verified the findings, and today, TB researchers are beginning to accept the new model of the mycobacterial cell envelope.
New Targets for TB
With the wall of resistance beginning to crumble, the Niederweis lab is studying the proteins in TB’s cell walls with the hopes that one might represent an antibiotic target. Antibiotics clear most bacterial infections within a few weeks, yet current drugs require six months to cure a TB infection. Niederweis believes that the outer membrane is one of the reasons for the prolonged drug treatment.
“All the putative drug targets inside the cell are protected by the outer membrane, so it’s very difficult to reach these targets,” he says. “What appears to be more promising are targets on the outside of the cell.” As a result, he says, future anti-TB drugs may be able to greatly reduce the duration of treatment for one of the world’s oldest and deadliest bacterial pathogens.