What Glutamate Can Teach Us About Depression, Schizophrenia, Cancer, and More
By Kathleen Yount
Glutamate is the incredible, edible neurotransmitter. This amino acid is found in chips, yogurt, and ice cream, as well as the much-maligned MSG. It is also the key ingredient that helps neurons communicate, learn, make memories, and perform other essential functions.
For years, scientists have kept an eye on glutamate, suspecting that it plays a role in several debilitating diseases. But only recently have they discovered how to do anything about it. UAB researchers are leading the way in studies that could bring new treatments and new hope for people suffering from depression, schizophrenia, and even brain cancer.
All Hail the King
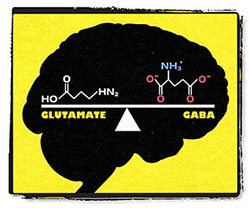 Glutamate and GABA are the king and queen of neurotransmitters. Glutamate stimulates neurons and GABA inhibits them in a delicate balancing act. Diseases such as depression, schizophrenia, and brain cancer are all associated with glutamate imbalances. Glutamate is the most abundant excitatory neurotransmitter, which means its job is to stimulate neurons. It works in tandem with GABA, the main inhibitory neurotransmitter, to maintain balance in the brain.
Glutamate and GABA are the king and queen of neurotransmitters. Glutamate stimulates neurons and GABA inhibits them in a delicate balancing act. Diseases such as depression, schizophrenia, and brain cancer are all associated with glutamate imbalances. Glutamate is the most abundant excitatory neurotransmitter, which means its job is to stimulate neurons. It works in tandem with GABA, the main inhibitory neurotransmitter, to maintain balance in the brain.
Glutamate and GABA are the king and queen of neurotransmitters. All others—including the more-famous serotonin, norepinephrine, and dopamine—have important functions of their own, but ultimately they serve to modulate the glutamate and GABA systems.
The brain’s glutamate balancing act revolves around a highly evolved system of molecules called receptors to which glutamate binds to produce actions in the brain’s cells. Another family of molecules called transporters mops up unused glutamate after it has been released. Much of the current research on glutamate dysfunction centers on these processes of give, take, and transport, to see if understanding the exchange can shed light on what happens when the glutamate system goes wrong, and how we might make it right again.
Rapid Relief for Depression
Richard Shelton, M.D., professor and vice chair of research in the UAB Department of Psychiatry and Behavioral Neurobiology, says that glutamate is an old suspect in the development of major depression. “We’ve known glutamate had a role in depression for some 20 years,” he says, but not enough was known to shift drug development away from the serotonin, dopamine, and norepinephrine systems, which have been the only therapeutic targets.
 Deficiencies in glutamate are suspected in diseases such as depression—and new drugs that target glutamate may help patients who have found little relief elsewhere. Then, in 2007, Carlos Zarate, M.D., a researcher at the National Institutes of Mental Health (NIMH), demonstrated that the drug ketamine, used as an anesthetic in hospitals and in veterinary medicine, could produce a rapid and robust antidepressive effect. And unlike classic antidepressant drugs, which can take several weeks to reach full effectiveness, ketamine yields results in two hours.
Deficiencies in glutamate are suspected in diseases such as depression—and new drugs that target glutamate may help patients who have found little relief elsewhere. Then, in 2007, Carlos Zarate, M.D., a researcher at the National Institutes of Mental Health (NIMH), demonstrated that the drug ketamine, used as an anesthetic in hospitals and in veterinary medicine, could produce a rapid and robust antidepressive effect. And unlike classic antidepressant drugs, which can take several weeks to reach full effectiveness, ketamine yields results in two hours.
This was exciting news, because ketamine is a drug that acts on glutamate, through a receptor called NMDA. One theory is that ketamine regulates the glutamate function in these patients, which reinvigorates the communication among existing neurons. If true, this could be a faster and perhaps more effective way to relieve depression symptoms than drugs that boost serotonin, such as the commonly prescribed SSRIs, which trigger new neuron formation.
Ketamine itself isn’t a realistic depression therapy strategy—in addition to being a potential drug of abuse (sometimes known as Special K), it can now only be delivered via IV infusion. But pharmaceutical companies are now designing drugs that target the same NMDA receptor, or subtypes of the receptor, in a way that will generate results similar to ketamine, without the side effects.
“It’s terribly exciting,” Shelton says. “The available antidepressant drugs haven’t really changed much since the 1950s—certainly they’re cleaned up, they have fewer side effects, but they’re still the same basic components.” Glutamate-targeting drugs could be a second chance for many patients who are currently out of options.
Drug Discovery for Schizophrenia
Like depression researchers, schizophrenia scientists have a longstanding interest in glutamate—and a frustration with available medications, which all act on the dopamine system and which provide no relief for many of the symptoms of this illness.
James Meador-Woodruff, M.D., chair of the psychiatry department at UAB, explains that dopamine became a treatment target after researchers observed that people taking dopamine activators (amphetamines) exhibit similar symptoms to people with schizophrenia.
Then, “when I was finishing my residency in the late 1980s, we started seeing lots of young people coming into the ER, looking for all the world like they had schizophrenia,” Meador-Woodruff says. They were all using PCP, which had flooded the street-drug market at that time. “PCP-induced psychosis is a much closer mimic of schizophrenia than the mania induced by amphetamines,” says Meador-Woodruff. And because PCP acts on glutamate, Meador-Woodruff says, he was convinced that the neurotransmitter was a key factor in schizophrenia.
|
“We know that there is something wrong with the handling of glutamate in the brains of people with schizophrenia. But is there too much glutamate? Too little? Is it in the wrong place at the wrong time?” |
But these observations only implicated glutamate as a player in the syndrome; they don’t show a mechanism by which it affects the disease. “We know that there is something wrong with the handling of glutamate in the brains of people with schizophrenia,” says UAB psychiatric researcher Robert McCullumsmith, M.D., Ph.D. “But is there too much glutamate? Too little? Is it in the wrong place at the wrong time?”
McCullumsmith and Meador-Woodruff have been investigating the glutamate question since they worked together at the University of Michigan. Today, they’re using samples of brain tissue from schizophrenia patients, provided by the Alabama Brain Collection at UAB in addition to samples from collections in New York and Washington, D.C., to try to piece together the processes.
The researchers have some evidence to suggest that the NMDA receptor and its neighboring AMPA receptor are key to the malfunction—not that there are too many or too few of them, but that they don’t end up where they need to be to process glutamate correctly. “We’re looking at several cellular processes that might help explain why,” says Meador-Woodruff. “That might mean that the ultimate target isn’t the glutamate itself, but the processes that affect its regulation—and that could open up a whole new world of drug discovery in psychiatry.”
Understanding the role of glutamate is not the same thing as understanding the cause of the problem, notes McCullumsmith. Schizophrenia is a syndrome—a collection of symptoms—not a one-cause-fits-all disease. “The very best genetic data show that schizophrenia is less heritable than bipolar disorder,” McCullumsmith says, “and there’s a huge environmental component that gets you there. There are probably two dozen ways to break your brain to the point of having glutamate malfunction that leads to schizophrenia.”
But if they can clarify the role of the glutamate system, even in a small percentage of patients, it would be a huge breakthrough. “Schizophrenia affects 1 percent of the population, which sounds small,” McCullumsmith says. “But that means in a high school class of 300, three students may develop schizophrenia. These are young people who had bright futures, who are now vulnerable to becoming unemployed, unemployable, even homeless.”
Glutamate and the Negative Symptoms and Cognitive Deficits of Schizophrenia
Adrienne Lahti, M.D., division director of behavioral neurobiology at UAB, is particularly interested in glutamate’s role in the early development of schizophrenia.
During that time, sometimes as much as a year before delusional symptoms begin, people can show real cognitive decline and even a decrease in overall gray matter volume in the brain. Research shows that glutamate levels rise during this period.
The hearing of voices, hallucinations, and paranoia are known as the “positive” symptoms of schizophrenia. But Lahti says that’s not the whole picture of the illness. The negative symptoms, including a lack of motivation, lack of self-grooming, and the cognitive deficits, such as attention and memory impairments, can be just as debilitating. “They can make holding a job impossible,” Lahti says.
There are no current treatments for schizophrenia’s negative symptoms and cognitive deficits. Even people who respond well to medication can continue to suffer them. “We want to know if the increasing levels of glutamate in the early stages of the disease are toxic to the brain”—and if that might be a factor in the negative symptoms, Lahti says.
 An overabundance of glutamate may play a key role in the development of schizophrenia. Lahti uses MR spectroscopy, a noninvasive way to get a general reading of glutamate levels in different regions of the brain, to test the theory that patients who respond to schizophrenia medications have an associated reduction in glutamate. She also recently opened a clinic specifically to serve patients in the earliest stages of schizophrenia. “Often when people first begin showing symptoms, parents don’t know what to do—and because of that, there’s a delay in seeking treatment,” Lahti says. “This is a very fragile time, and the stakes are high. Any breakthrough could make a dramatic difference in the lives of these young people.”
An overabundance of glutamate may play a key role in the development of schizophrenia. Lahti uses MR spectroscopy, a noninvasive way to get a general reading of glutamate levels in different regions of the brain, to test the theory that patients who respond to schizophrenia medications have an associated reduction in glutamate. She also recently opened a clinic specifically to serve patients in the earliest stages of schizophrenia. “Often when people first begin showing symptoms, parents don’t know what to do—and because of that, there’s a delay in seeking treatment,” Lahti says. “This is a very fragile time, and the stakes are high. Any breakthrough could make a dramatic difference in the lives of these young people.”
Gliomas: Too Much of a Good Thing
The toxic effects of too much glutamate also seem to play a role in a very different problem, born in the glial cells that make up at least half—and perhaps much more—of our brains. While the exact role of glial cells is still under investigation, it’s clear that they are the source of gliomas, the deadliest of brain cancers. “In the dense landscape of the skull, full of brain tissue, there is no breathing room for a growing tumor,” says UAB glial cell biologist Harald Sontheimer, Ph.D. So gliomas must destroy tissue to make room for themselves.
And it turns out that they use glutamate to do it. Gliomas release hundreds of times the normal amount of glutamate into surrounding tissue. This overstimulates the nearby neurons, at first causing seizures; as it continues it ultimately kills the neurons. Eighty percent of patients with gliomas will suffer from at least one seizure, and some will develop tumor-associated epilepsy. “I was watching the news about Ted Kennedy’s seizure in May 2008,” Sontheimer says. “I said to my wife, ‘He has a glioma’—and sure enough, a few months later, the news came that he was diagnosed with cancer.”
Sontheimer directs UAB’s Center for Glial Biology in Medicine—one of the few centers in the world that focuses exclusively on these dense cells of the brain. He thinks there’s a way to block gliomas from their toxic torrent of glutamate and perhaps to slow or stop their growth.
Just like in neurons, glutamate moves in and out of glial cells through a complex system of receptor and transporter molecules. One transporter molecule, xc antiporter, acts as a sort of revolving door—it lets glutamate out and takes in cystine, another amino acid. Sontheimer’s team looked for compounds that could bind to this transporter molecule and essentially jam a wedge into the door. They came up with several possible compounds—one matching the structure of the existing drug sulfasalazine, which is used to treat Crohn’s disease and ulcerative colitis.
Sulfasalazine was approved by the FDA in 1950, which means that it has been safely used by hundreds of thousands of people. Within months of publishing this discovery, Sontheimer was working with UAB neurologist Burt Nabors, M.D., on a phase 1 study to determine the efficacy of the drug in reducing glutamate in the brain. He’s also collaborating with Lahti to use MR spectroscopy to measure glutamate levels. If they drop after patients take sulfasalazine, researchers will look at how the patient’s symptoms and disease progression change (or don’t).
The researchers have lots of questions to answer, including whether sulfasalazine could ever get into the brain effectively. They already know that about 80 percent of the drug gets metabolized in the gut. (It is designed for gastrointestinal diseases, after all.) “But in theory,” Sontheimer says, “if you could use sulfasalazine or a drug like it to prevent the neuron kill, you could slow or even stop the growth of the tumor.” And the patient would have fewer or no seizures, which can be among the more debilitating symptoms of patients with these types of tumors.
“It’s so exciting because it’s such a quick path to patients,” says Sontheimer. “From the publication date to the first patient enrollment was just a few months, instead of the years it would usually take.”