The hypothalamic suprachiasmatic nucleus (SCN) is the master clock responsible for controlling circadian rhythms in mammals1. These rhythmic cycles generated by the SCN are entrained to the environmental light/dark cycle via photic signals from intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin. In addition to their critical role in nonimage-forming vision, ipRGCs also project to the lateral geniculate nucleus (LGN) to modulate conscious vision, and the pretectal olivary nucleus (PON) to control pupil constriction2, 3, 4. Orexin is a neuropeptide that regulates arousal, wakefulness, and appetite. Orexingeric cells in the posterior hypothalamus receive input from the circadian system5. It has been reported that orexin is present in melanopsin-expressing ipRGCs6, 7. However, most knowledge of these cells has been gained from studies on nocturnal rodents, whose visual systems are distantly related to those of humans and other diurnal primates. Injection of cholera toxin B (CtB), an anterograde axonal tracer, was used to visualize retinal projections in the brain, and anti-orexin antibodies were used to determine if these projections also contained orexin.
Authors:
Treffalls, J. A.1
Chang, K. Q.2
Gamlin, P. D.1,3,4,5
Departments
- Department of Ophthalmology, University of Alabama at Birmingham School of Medicine, Birmingham, AL, USAVision Sciences Graduate Program, University of Alabama at Birmingham, Birmingham, AL, USA
- Department of Neurobiology, University of Alabama at Birmingham School of Medicine, Birmingham, AL, USA
- Department of Psychology, University of Alabama at Birmingham, Birmingham, AL, USA
- Department of Biomedical Engineering, University of Alabama at Birmingham, Birmingham, AL, USA
Introduction
The hypothalamic suprachiasmatic nucleus (SCN) is the master clock responsible for controlling circadian rhythms in mammals1. These rhythmic cycles generated by the SCN are entrained to the environmental light/dark cycle via photic signals from intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin. In addition to their critical role in nonimage-forming vision, ipRGCs also project to the lateral geniculate nucleus (LGN) to modulate conscious vision, and the pretectal olivary nucleus (PON) to control pupil constriction2, 3, 4. Orexin is a neuropeptide that regulates arousal, wakefulness, and appetite. Orexingeric cells in the posterior hypothalamus receive input from the circadian system5. It has been reported that orexin is present in melanopsin-expressing ipRGCs6, 7. However, most knowledge of these cells has been gained from studies on nocturnal rodents, whose visual systems are distantly related to those of humans and other diurnal primates. Injection of cholera toxin B (CtB), an anterograde axonal tracer, was used to visualize retinal projections in the brain, and anti-orexin antibodies were used to determine if these projections also contained orexin.
Materials and Methods
Animals
One male macaque monkey, aged 20 years, was used for the tracer study. The animal was kept in the primate animal facility of the University of Alabama at Birmingham under 12:12-hour light:dark conditions. For the tracer injection, the animal received bilateral injections of 100 μl of 0.5% CtB (List Biological Laboratories, Campbell, CA) conjugated with either Alexa Fluor 488 (right eye) or biotin (left eye) in sterile water into the vitreous body with a 1-cc tuberculin syringe under isoflurane. Experimental procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC) and complied with the USPHS policy on humane care and use of laboratory animals.
Tissue Preparation
After a survival time of 6 days, the animal was deeply anesthetized with Ketamine followed by terminal anesthesia with Pentobarbital (200 mg/kg BW). The animal was then perfused through the aorta with 2 L 1% Sodium nitrite/0.9% Sodium chloride, and fixed with 4 L of 4% paraformaldehyde in 0.1 M phosphate buffer. The brain was removed, stereotaxically blocked in the coronal plane at the level of the brainstem, and was placed in 30% sucrose in 0.1 M PB for 5 days. The brain was then sectioned at 30-34 μm on a freezing, sliding microtome (AO 860). All sections from the level of the optic chiasm to the posterior superior colliculus were collected individually into 0.1 M phosphate buffer in 24-well tissue culture plates for later processing. Retinas from all eyes were processed as previously described8. The anterior portion of the globe, lens, and vitreous were removed.
Immunohistochemistry and Microscopy - CtB
Free-floating brain sections were rinsed in 0.1 M PBS and then incubated in 0.3% hydrogen peroxide in PBS for 20 minutes to quench endogenous peroxidase activity. Sections were then blocked in 10% streptavidin and biotin blocking solution (Vector laboratories) for 30 minutes each. Sections were rinsed in 0.1 M PBS, then blocked in 2% normal goat serum and NHP serum and 0.5% Triton X-100 in 0.1 M PBS for approximately 12 hours at 4° C. The sections were then incubated for 5 days at 4° C in a primary antibody, goat anti-CtB (List Biological Laboratories, 1:2000) in a 2% rabbit serum/NHP serum/ 0.1 M PBS/1% Triton X-100. Sections were then rinsed in 0.1 M PBS and incubated for 4 hours in biotinylated rabbit anti-goat IgG (Vector Laboratories, 1:500) in 0.1 M PBS/0.5% Triton X-100. Following rinsing, sections were incubated for 2 hours in streptavidin-HRP (Rockland, 1:1000) in PBS plus 0.5% Triton X-100. They were then rinsed and reacted with 3’ 3’ diaminobenzidine (DAB), Pierce metal-enhanced solution for 12 minutes. Sections were rinsed, mounted on gel-subbed slides, and allowed to dry. They were then cleared in Xylene, and cover-slipped with Permount. NHP brain sections were imaged an Olympus BH-2 microscope at 4x/NA 0.17. Images of the parvocellular and magnocellular layers of the LGN were generated in Adobe Photoshop as stitched montages of four separate images. Contrast and brightness of images were adjusted in Photoshop for optimal clarity.
Immunohistochemistry and Microscopy - Orexin
Cryosections of retinas were rinsed with 0.1 M PBS, incubated in 0.5% Triton X-100, and blocked in streptavidin and biotin blocking solution (Vector Laboratories) and 2% NGS/NMS/0.1 M PBS. Retinal sections were then incubated in primary antibodies raised against orexin (rabbit polyclonal, Phoenix Pharmaceuticals, H-003-30, 1:2000, or Millipore, PC345 (14-33), 1:500), in a solution containing 2% NGS/NMS/0.1 M PBS for approximately 12 hours at 4° C. Samples were then rinsed in 0.1 M PBS and incubated for 1 hour at room temperature with a secondary antibody, biotinylated goat anti-rabbit (Vector, 1:500) in 0.5% Triton X-100. Following another rinse, samples were incubated for 1 hour at room temperature streptavidin conjugated with Alexa-Fluor 594 (Molecular Probes, 1:200) in 0.5% Triton X-100. After a final rinse in 0.1 M PBS, an aqueous based anti-fade media was applied (Prolong Gold, Life Technologies) and slides were cover-slipped. Brain sections were stained following the procedure above, with longer incubation times to allow for penetration of thicker sections. Fluorescent images were captured on an A1R confocal microscope (Nikon) using the following objectives: 10x/NA 0.3 WD, 20x/NA 0.75 WD, 40x Oil Immersion/NA 1.30 WD.
Results
CtB-immunoreactive retinal projections were found throughout the macaque brain, indicating successful uptake and movement of the tracer to downstream nuclei such as the LGN, SCN, pregeniculate nucleus, and PON (Figure 1A-D). Alexa Fluor-488 fluorescent RGCs (Figure 1E) serve as a positive control for the immunohistochemical staining in the brain.
Figure 1 | CtB transport in central retinal projections
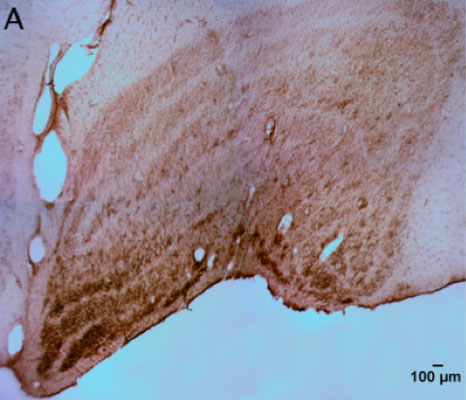
Figure 1-A

Figure 1-B
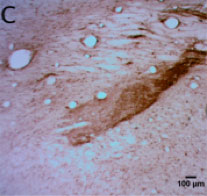
Figure 1-C
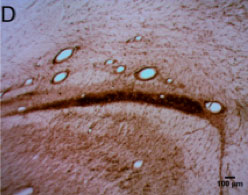
Figure 1-D
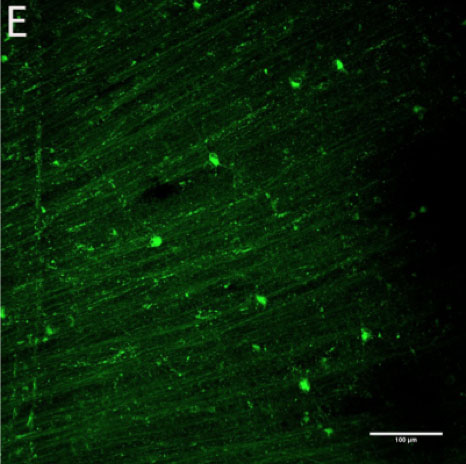
Figure 1-E
Potentially orexin-immunoreactive ganglion cells were identified in the macaque retina (Figure 2B, D) using two polyclonal antibodies (Phoenix and Millipore). The hypothalamus (Figure 2A, C) served as a positive control for tagging of orexin in the retina because orexinergic neurons in the hypothalamus have been previously confirmed.
Figure 2 | Presence of orexin staining using two primary antibodies at optimal working concentrations
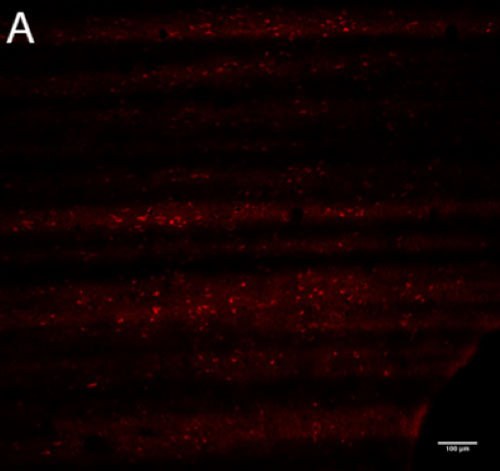
Figure 2-A
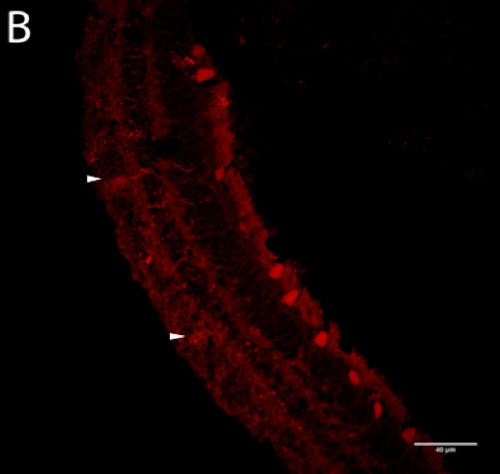
Figure 2-B
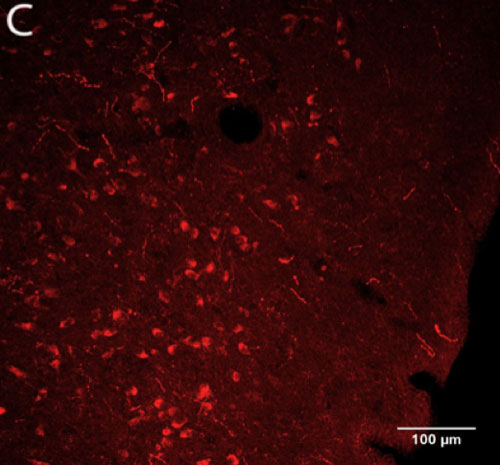
Figure 2-C
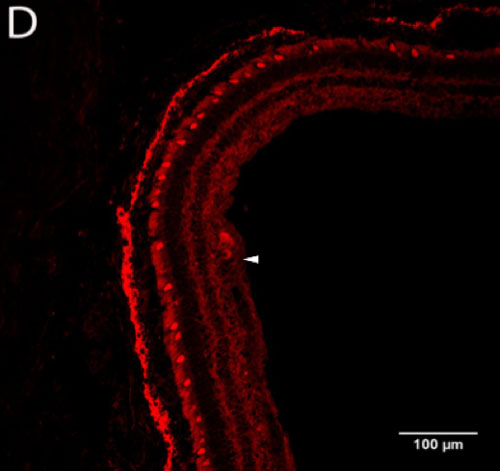
Figure 2-D
Discussion
HRP-immunoreactive neurons in the various vision processing nuclei provide evidence that CtB transports well from retina to the brain. However, transport may be selective for RGCs with larger soma such as parasol cells while excluding smaller bodied midget cells. There was strong staining in the SCN, PON, pregeniculate nucleus, and the magnocellular layers of the LGN. However, staining of the parvocellular layers of the LGN, which receive input from midget ganglion cells, was light. Preliminary evidence suggests limited orexin labeling of macaque RGCs, but future immunohistochemical analysis is necessary to confirm this finding. Cells within the oculomotor and trochlear nuclei were labeled (not pictured) – this could be due to either nonspecific immunohistochemical labeling or to leakage of CtB from the eye and uptake by the extraocular muscles.
Future directions of this study include the red-green approach for right and left eye. By using an anti-Alexa Fluor-488 primary antibody and secondary antibody conjugated with Alexa Fluor, it is possible to amplify the green fluorescent signal. Additionally, using streptavidin conjugated with Alexa Fluor-594 will label the biotin-CtB injected into the animal’s left eye. Other necessary experiments include the double labeling for orexin (Alexa-594) and CtB-Alexa 488 to determine if retinal projections in LGN or SCN may be orexinergic. Due to apparent nonspecific labeling of CNS neurons with Goat anti-CtB antibody troubleshooting with antibody concentrations and blocking solutions will be performed.
In response to possible positive data, double labeling of retinal sections for melanopsin or PACAP and orexin will be performed to determine if ipRGCs may also express orexin. Photoreceptors and the retinal pigment epithelium may auto-fluoresce, in future immunohistochemical protocols, quenching solutions will be applied to minimize artefactual fluorescence. Finally, a nuclear counter stain (DAPI) will also be used in retinal imaging to localize cell somas and confirm presence of orexin in RGCs.
References
- Bernard, S., Gonze, D., Čajavec, B., Herzel, H., & Kramer, A. Synchronization-induced rhythmicity of circadian oscillators in the suprachiasmatic nucleus. PLoS Computational Biology 3(4), e68 (2007).
- Fu, Y., Liao, H.-W., Do, M. H., & Yau, K.-W. Non-image-forming ocular photoreception in vertebrates. Current opinion in neurobiology 15(4), 415-422 (2005).
- Gamlin, P. D., McDougal, D. H., Pokorny, J., Smith, V. C., Yau, K. W., & Dacey, D. M. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision research 47(7), 946-954 (2007).
- Do, M. T. H., & Yau, K. W. Intrinsically photosensitive retinal ganglion cells. Physiological reviews 90(4), 1547-1581 (2010).
- Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., ... & Arch, J. R. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92(4), 573-585 (1998).
- Savaskan, E., Müller-Spahn, F., Meier, F., Wirz-Justice, A., & Meyer, P. Orexins and Their Receptors in the Human Retina. Pathobiology 71(4), 211-216 (2004).
- Liu, F., Xu, G. Z., Wang, L., Jiang, S. X., Yang, X. L., & Zhong, Y. M. Gene expression and protein distribution of orexins and orexin receptors in rat retina. Neuroscience 189 (2011).
- Hannibal, K., Strang, C. E., Peterson, B. B., Dacey, & Gamlin, P. D. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. Journal of Comparative Neurology 522(10), 2231-2248 (2014).
