New Research Unit Expands Access to Cutting-Edge Treatments at UAB
By Matt Windsor
 Lynn Holt (above, with his wife, Suzy) is receiving a new treatment for brain cancer as part of a phase 1 clinical trial at UAB. Phase 1 trials are the first studies of new treatments conducted in humans. UAB's new Phase 1 Clinical Trials Unit, which opened in February 2013, will allow more patients to take part in these potentially lifesaving trials, and increase the number of phase 1 trials available at UAB.In one six-month stretch last year, Lynn and Suzy Holt got enough bad news to last a lifetime. Suzy found out she had breast cancer in June 2012, just after Lynn had left a longtime job as a food distributor to start his own business. Then, as Suzy was in the middle of treatment in early October, Lynn was diagnosed with glioblastoma multiforme, an aggressive form of brain tumor. "I was a stage 4," he says, which means the tumor was spreading quickly. "My doctor said, 'You need to go to UAB. This needs to be handled by the experts.'"
Lynn Holt (above, with his wife, Suzy) is receiving a new treatment for brain cancer as part of a phase 1 clinical trial at UAB. Phase 1 trials are the first studies of new treatments conducted in humans. UAB's new Phase 1 Clinical Trials Unit, which opened in February 2013, will allow more patients to take part in these potentially lifesaving trials, and increase the number of phase 1 trials available at UAB.In one six-month stretch last year, Lynn and Suzy Holt got enough bad news to last a lifetime. Suzy found out she had breast cancer in June 2012, just after Lynn had left a longtime job as a food distributor to start his own business. Then, as Suzy was in the middle of treatment in early October, Lynn was diagnosed with glioblastoma multiforme, an aggressive form of brain tumor. "I was a stage 4," he says, which means the tumor was spreading quickly. "My doctor said, 'You need to go to UAB. This needs to be handled by the experts.'"
Lynn, who describes himself as a "real online kind of guy," had done his research and knew what he was up against. Glioblastoma multiforme is the deadliest type of brain cancer, with an average survival rate of less than 15 months from diagnosis. "I wanted every hope I could get," he says.
Burt Nabors, M.D., director of UAB's Division of Neuro-Oncology, had something to offer. Nabors recommended the standard treatment—neurosurgery to remove as much of the tumor as possible, followed by radiation therapy and then chemotherapy. But Nabors also told Holt about BSI-201. This experimental "molecularly targeted therapy" is designed to work with existing chemo drugs to improve the destruction of cancer cells. It is being studied in a phase 1 trial at UAB and other institutions that are part of the National Cancer Institute's Adult Brain Tumor Consortium.
The small-scale study enrolled only 40 patients nationwide, including 10 at UAB. "I was the last person to get in, and I'm blessed to get this drug," Holt says. "It's really doing well." As of early July 2013, when he was nearing the end of the treatment cycle, Holt hadn't experienced any tumor regrowth.
New Room for Research
More patients at UAB will be able to access such cutting-edge treatments with the February 2013 opening of the Phase 1 Clinical Trials Unit. The new space, run by the UAB Center for Clinical and Translational Science (CCTS), is a nearly 8,000-square-foot expansion of the center's existing Clinical Research Unit, effectively doubling its capacity by adding five more treatment rooms and another infusion suite to the unit.
(Story continues beneath the image)
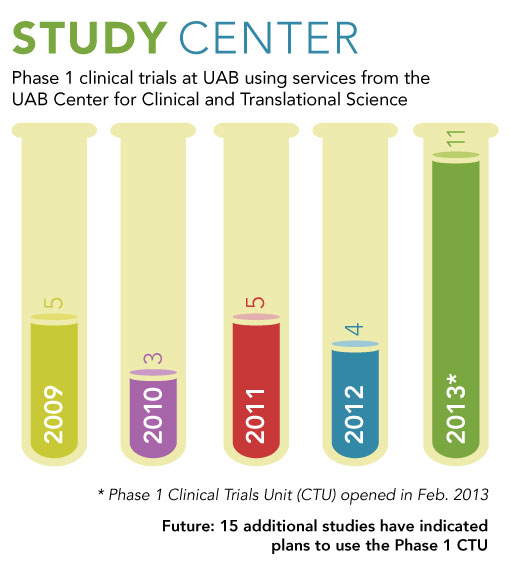
The facility "puts us in a more proactive position in terms of recruiting phase 1 studies to UAB," says Robert Kimberly, M.D., CCTS director and senior associate dean for research at the UAB School of Medicine. "By adding this facility, we are adding capacity for additional studies. And by being involved from the very beginning as new compounds make the transition to human use, we are in the position of being able to offer these therapies to our patients."
First-in-Human Studies
Phase 1 trials are the first chance for researchers to test promising treatments in humans, Kimberly explains. They are also a crucial step in the long process that leads from lab testing to a final product approved for clinical use by the U.S. Food and Drug Administration. (To learn more about the phases of clinical trials, see "On Trials.")
There is no guarantee of success; many treatments that were promising in the lab or in animal models fizzle out once they reach human testing. At this point, "the most we can say about BSI-201 is that the drug appears to be safe when combined with standard treatment for brain cancer," says Nabors, who is the medical director of the Clinical Research Unit.
But for patients like Holt, a phase 1 treatment can offer new hope in the face of a chilling diagnosis—years before the drug becomes clinically available. And by helping himself, Holt also is helping countless others, Nabors explains. "Today's standard therapies were once in phase 1 trials," he says. "Someone had to take part in the trials of Temodar [the frontline chemotherapy drug for brain cancer] before it became the standard treatment."
A Challenging Process
Many institutions would like to increase their participation in phase 1 trials. But they are a complicated, labor-intensive proposition, Kimberly points out. "These trials are the first time a drug has been used in humans," he says. "You have best guesses from preclinical testing, but you need to know how the body will respond." To do that, researchers focus on pharmacokinetics (the study of how a drug moves through the body over time) and pharmacodynamics (the relationship between drug dose and its effects on the body).
|
Join In To learn more about accessing UAB's Phase 1 Clinical Trials Unit, contact Jolene Lewis at jelewis@uabmc.edu or (205) 934-7817. There are several other ways to take part in research at UAB: 1. Check the list: Each week, new studies are added to the Clinical Trials page maintained by UAB's official newspaper, the UAB Reporter. 2. Sign up: UAB participates in ResearchMatch, a free national volunteer recruitment registry that connects people looking to take part in research studies with investigators searching for participants. 3. Get the big picture: ClinicalTrials.gov, a site maintained by the National Institutes of Health, lists more than 150,000 publicly and privately funded human studies offered at locations around the world. Include "UAB" in a site search to narrow the results to studies at the university. |
This crucial process, known by the shorthand PKPD, requires frequent blood samples; specialized equipment such as mass spectrometers, which measure the masses and concentrations of atoms and molecules; and novel screening methods. "You need to have sophisticated support staff who are accustomed to doing these types of studies," Kimberly says. "Analysis of a new drug isn't routine—obviously, because it hasn't been in human use yet."
The staff and facilities at the new Phase 1 Clinical Trials Unit should give Kimberly and other UAB representatives an advantage as they actively recruit more phase 1 trials from pharmaceutical companies and other research groups, he says. They should also help keep more UAB-developed compounds in Birmingham when the drugs reach clinical testing. "When compounds are developed here, we have to make the case that it's appropriate for them to be tested in the phase 1 unit here," Kimberly says.
Drips and Donuts
Twice a week for the past six months, Holt has been coming to UAB's Jefferson Tower to receive BSI-201 through an infusion. He parks in a reserved lot just across the street and takes an elevator directly to the phase 1 unit on the 15th floor.
The facility, with its sweeping views of Red Mountain and private treatment rooms, is a dramatic change from the old location on a lower floor of the building, where patients received transfusions in an open room "with six chairs lined up next to each other," Holt recalls. "When they first brought us up here, I said, 'Whoa.' I was very impressed."
As he receives his remaining treatments, Holt sits in a recliner, talking with family members. The infusions take about an hour, he says. "They bring it in, plug it in, and I just sit here, eat a donut, and get a cup of coffee." To show his appreciation, Holt often brings a box of donuts for the staff, too.
After the infusions, Holt remains while nurses monitor him for any side effects. Meanwhile, his blood is being tested to follow the path BSI-201 takes through his body. He doesn't mind the wait; it gives him a chance to catch up with his wife and plan a beach getaway. "We were in chemo side by side in 2012," Suzy Holt says. "But we say 2013 will be our lucky year."
On TrialsTreatments must go through three stages of clinical trials before they can be approved by the U.S. Food and Drug Administration (FDA), says Robert Kimberly, M.D., director of the UAB Center for Clinical and Translational Science and senior associate dean for research at the UAB School of Medicine. |