We conduct the Fiscal Approval Process (FAP) to ensure a reliable and consistent method of evaluating clinical services identified by the department as routine care, the associated billing to third party payers is appropriate, and an efficient method to provide departments with research fees for clinical billable services. All studies containing UAB Health System clinical billable services (whether services are billed to study/sponsor or to insurance, as standard of care) should be submitted to CBR for review. Please see below two workflows for an overview of the institutional billing process for new studies and amendments, which includes the entities involved, and the order in which components occur.
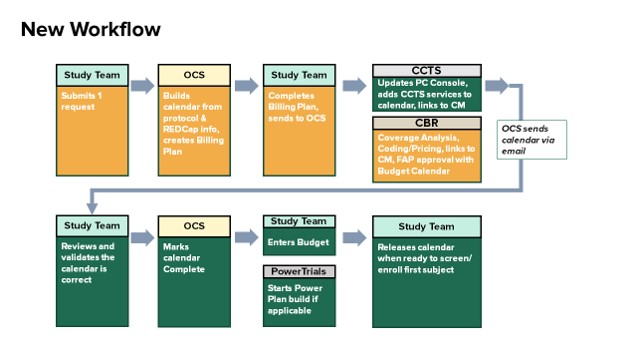
Overview of Billing Process (Final.05.29.20)
Overview of Billing Process -Amendments (Final.05.29.20)
OnCore Calendar (via RedCap) - The "front-door" for clinical trial submissions
(Contact OnCore Services at 4-888 or oncore@uabmc.edu )
If a study has UAB Health System clinical billable services, an OnCore Calendar is required, and the study is required to be managed in the OnCore system. The study team will submit an electronic submission for the Oncore Calendar, CBR review, and/or CCTS Services. The OnCore Calendar will be built first, and if there are UAB Health System clinical billable services, the calendar information will be forwarded by OnCore Calendar Services (OCS) automatically for CBR Review.
Processes
-
Full Fiscal Approval Process Review Submission — Required for any clinical trial/research project that includes clinical activities that are provided and billed by the UAB Health System.
-
Device Trial Submission — Required for any clinical trial/research project that includes clinical activities that are provided and billed by the UAB Health System.
-
Feasibility Fee Request Submission — Obtain preliminary research fees for protocol-required clinical billable activities that are provided and billed by the UAB Health System.
-
Amendment Review — Submit when protocol-driven clinical billable activities are affected by the study change or changes to the SiteMinder budget are indicated.
-
Ancillary Research Information — Services provided by ancillary research areas (non-clinical billable services).