 Maria Grant, MD, along with her team at the UAB Heersink School of Medicine Department of Ophthalmology and Visual Sciences teamed up with researchers from the University of Florida College of Medicine (Drs. Qiuhong Li, Mohan Raizada, and Bruce Stevens), the Mazankowski Alberta Heart Institute (Dr. Gavin Oudit), and Wright Labs, LLC, (Drs. Regina Lammendella and Justin Wright), for a study examining components of systemic and intestinal renin-angiotensin system (RAS) on gut barrier permeability and progression of diabetic retinopathy in human subjects and tested their hypotheses in mouse models of type 1 diabetes. Their study titled, “Maintenance of Enteral ACE2 Prevents Diabetic Retinopathy in Type 1 Diabetes,” was recently published in Circulation Research and gives hope for a new method of preventing and treating diabetic retinopathy, a diabetes complication which causes vision loss due to damaged blood vessels in the retina, or light-sensitive tissue at the back of the eye. The researchers found a correlation between increased gut permeability and severity of diabetic eye disease giving weight to the claim that gut health is related to eye health.
Maria Grant, MD, along with her team at the UAB Heersink School of Medicine Department of Ophthalmology and Visual Sciences teamed up with researchers from the University of Florida College of Medicine (Drs. Qiuhong Li, Mohan Raizada, and Bruce Stevens), the Mazankowski Alberta Heart Institute (Dr. Gavin Oudit), and Wright Labs, LLC, (Drs. Regina Lammendella and Justin Wright), for a study examining components of systemic and intestinal renin-angiotensin system (RAS) on gut barrier permeability and progression of diabetic retinopathy in human subjects and tested their hypotheses in mouse models of type 1 diabetes. Their study titled, “Maintenance of Enteral ACE2 Prevents Diabetic Retinopathy in Type 1 Diabetes,” was recently published in Circulation Research and gives hope for a new method of preventing and treating diabetic retinopathy, a diabetes complication which causes vision loss due to damaged blood vessels in the retina, or light-sensitive tissue at the back of the eye. The researchers found a correlation between increased gut permeability and severity of diabetic eye disease giving weight to the claim that gut health is related to eye health.
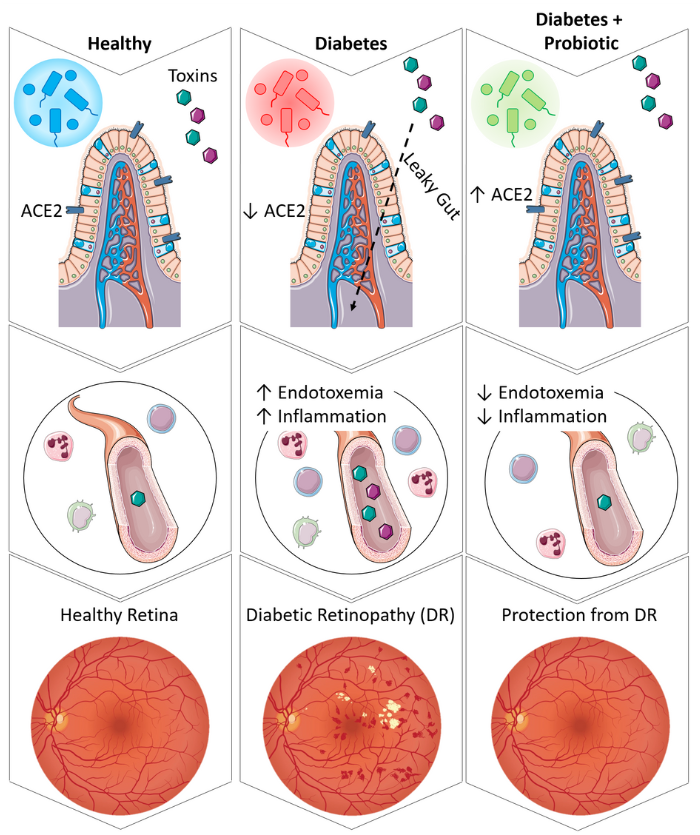
The RAS is a hormone system which regulates blood pressure, cell growth, fluid and electrolyte balance, cardiovascular remodeling, and responding to your body’s needs. For example, when the RAS is overactive, blood pressure will be too high. On the other hand, when the RAS is not active enough, blood pressure will be too low. The study by Prasad et. al. emphasizes the importance of the gut-eye axis and this team of researchers set out to determine its role in diabetic retinopathy and how they can positively affect this axis.
In their study, Prasad et. al. collected data on human patients with diabetic retinopathy as well as diabetics without retinopathy and healthy control subjects. The researchers found in this human cohort that individuals with diabetic retinopathy also had systemic RAS dysfunction that led to increased gut permeability and endotoxemia. Increased gut permeability and endotoxemia cause more toxins and other bacterial products to leave the digestive system and enter the bloodstream. These elevated levels of toxins in the bloodstream cause damage to the vasculature and in particular the vessels of the eye.
In their animal models, the researchers gave diabetic mice a probiotic that improved gut health. They used a bacterium which generates a specific enzyme, angiotensin converting enzyme 2 (ACE2), that supports the protective RAS in the intestines, blood, and eye. Dr. Quihong Li at University of Florida developed this probiotic. The probiotic strengthened the intestinal barrier, protecting it from damage, and subsequently reducing glucose absorption. This reduced glucose absorption allowed the RAS to act normally and maintain a healthier gut and reduced systemic inflammation. The researchers discovered the mice with stronger gut health were protected from developing diabetic retinopathy. More promising, they found diabetic gut and retinal pathology could was reversed when present in these mice.
Further research is needed in a human cohort to determine if this novel ACE2-boosting probiotic can be used in humans and garner the same results, but researchers are hopeful that using this approach to improving gut health will bring a new method of preventing and treating diabetic retinopathy to the public.
Prasad R. et al, 2022. Maintenance of Enteral ACE2 Prevents Diabetic Retinopathy in Type 1 Diabetes. Circulation Research, PMID: 36448480 doi: 10.1161/circresaha.122.322003