 I am going to expand on the excellent introduction Hannah gave you to the science of ocean acidification (OA) in an earlier blog. In doing so, I will highlight some of the bigger picture implications of ocean acidification globally. I will focus on two different cold-water geographic regions of the world and for each briefly highlight the impacts of ocean acidification on key, ecologically or economically (or both) important marine organisms. Both regions are ‘hot spots’ for ocean acidification.
I am going to expand on the excellent introduction Hannah gave you to the science of ocean acidification (OA) in an earlier blog. In doing so, I will highlight some of the bigger picture implications of ocean acidification globally. I will focus on two different cold-water geographic regions of the world and for each briefly highlight the impacts of ocean acidification on key, ecologically or economically (or both) important marine organisms. Both regions are ‘hot spots’ for ocean acidification.
But first a little more chemistry. You will recall that Hannah talked about how the world’s oceans are absorbing about one third of the carbon dioxide we are adding to the atmosphere from burning fossil fuels. In so doing, our oceans have become about thirty percent more acidic than they were at the onset of the industrial revolution. When it comes to the chemistry of ocean acidification, in addition to the increase in free hydrogen ions in seawater that Hannah focused on in her blog, there is yet another chemical process that occurs when carbon dioxide dissolves in seawater. I will not detail the chemistry but suffice it to say that the result is that the building blocks of shells, calcite and aragonite are becoming less ‘available’ to animals living in seawater. Right now, these shell-building blocks are cheap to acquire - as their levels in sea water are high (known as saturated levels). But ocean acidification is changing this. Shelled marine organisms are going to have to expend energy that might have gone to growth or reproduction just to secure the ‘bricks’ to build and maintain their shells.
In the big picture, all oceans are experiencing acidification, but not necessarily equally. For example, the Southern Ocean surrounding Antarctica is known to have among the highest levels of acidification due to the simple relationship between water temperature and the solubility of atmospheric carbon dioxide. The colder the water the more carbon dioxide is absorbed.
In our global exploration of important prospective impacts of ocean acidification, let’s start by focusing on Antarctic seas. Just as amphipods are key organisms when it comes to the ecology of seaweed communities (Hannah will talk more about this in a future blog), there are two other key groups of Antarctic marine organisms that come to mind when considering the impacts of ocean acidification on polar life – these are the shelled sea butterflies and shrimp-like krill.
Shelled sea butterflies (small swimming snails also called pteropods) are about the size of a pencil’s eraser but are seemingly as abundant as the stars. The opening image is a greatly magnified representative. There are so many of them that they are considered an important component of the global carbon cycle. And when they die their shells sink to the sea floor and become a soft sediment known as ‘pteropod ooze.’ Unfortunately, studies have shown that in the Southern Ocean shelled pteropods collected from the sea are already showing signs of dissolution of their delicate aragonitic shells. In a recent 2021 laboratory study the shells of the sub-antarctic pteropod Limacina retroversa were smaller and less dense when grown in near-future levels of ocean acidification. It is likely that shelled pteropods are suffering from both dissolution of the shell and the increased costs of building and maintaining a shell.
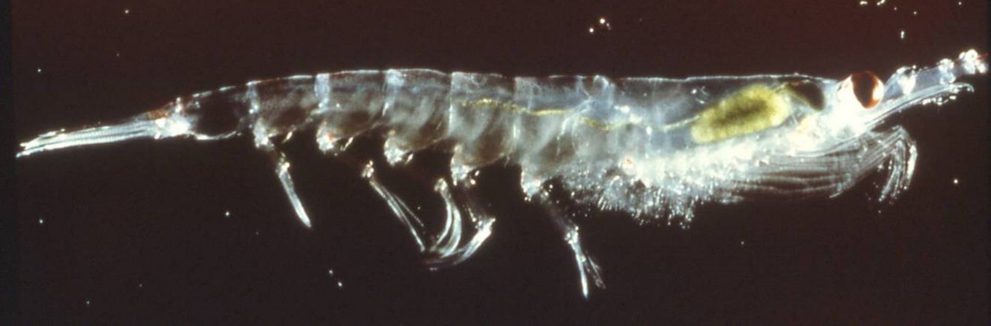
Studies of the potential impacts of ocean acidification on Antarctic krill have been more promising in terms of their apparent resistance to near-future ocean acidification. Very few impacts of ocean acidification have been reported on adults of Antarctic krill. For example, in a recent 2018 study, scientists found adults of the most common Antarctic krill, Euphausia superba (imaged above), were able to survive, grow, store fat, and maintain respiration rates in near-future ocean acidification conditions. However, in at least one earlier study, investigators found that egg hatching and larval development in Euphausia superba may be impeded by near-future levels of ocean acidification. The picture for Antarctic krill is hopeful but still clouded. The answer to this riddle is critical as krill are the foundation of Antarctic food webs.

The second geographic region I will focus on known as a ‘hot spot’ for ocean acidification is the Pacific coast of North America. Here, deep offshore waters rich in carbon dioxide with low pH are seasonally lifted to the surface by currents in a process known as ‘upwelling.’ As the human combustion of fossil fuels is increasing levels of carbon dioxide in the Pacific Ocean, seasonal upwelled waters are increasingly challenging marine life. The most famous examples of this challenge are oysters grown on farms along the coasts of California, Oregon, and Washington. Oyster farmers who routinely pump water directly from the sea to grow spat (baby oysters) began to experience catastrophic die-offs of their oyster stock. At first, the farmers suspected water pollutants were responsible, but before long they noticed that the oyster spat die-offs always occurred coincident with coastal upwelling events. It turned out that ocean acidification was the culprit!

Today, oyster farmers have addressed the issue by installing alarms to warn them of acidic seawater events and when needed, switching to seawater stored in tanks to prevent the kills. Importantly, they also voiced their deep concerns about ocean acidification and its impacts on their future profession to members of Congress.
In summary, ocean acidification is a big-picture global problem – and among the world’s marine organisms, there will be winners and losers. Hannah will soon be telling you more about our experimental ocean acidification setup at Palmer Station and the experiments we will do with winners and losers.