An Early Warning Sign for Inflammation
|
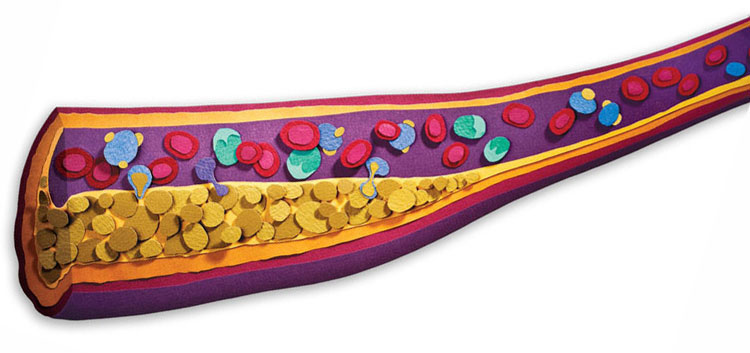
Canary or Culprit?“Research has established that high levels of inflammation are strongly predictive of heart disease, and that lowering inflammation can prevent heart attacks and other cardiovascular events,” says Alexander J. Szalai, Ph.D., professor in the Division of Clinical Immunology and Rheumatology. Szalai has spent 20 years studying the biology of one of the most hotly debated subjects in heart disease: C-reactive protein (CRP). CRP is what is known as an acute-phase reactant, which means it floods into the bloodstream in response to infection, injury, and inflammation. Most of the time, Szalai explains, CRP in the blood is a bit like a horse grazing in a field—part of the landscape, but fairly quiet. That is, until it is triggered by inflammatory processes, at which point “that horse begins to run very fast.” CRP levels can skyrocket overnight from normal readings of one to three milligrams per liter of blood to more than 500 mg/L in the case of a severe infection or burn. Because of its dynamic range—and because it is fairly easy to measure with standard blood tests—“CRP is a good gauge of underlying inflammation in the body,” says Szalai. Scientists have learned much about CRP in the last two decades, including uncovering a significant link between high levels of the protein in a person’s bloodstream and development of atherosclerosis and restenosis, which occurs when blood vessels opened in a procedure known as angioplasty begin to narrow again and block the normal flow of blood. (Story continues below image) |
 |
|
Inflammation is one of the leading causes of atherosclerosis, the narrowing of the arteries that leads to many heart attacks and strokes. Research at UAB and elsewhere is focusing on a promising target: good cholesterol. Read more
Researchers are still debating whether CRP is “actually helping fuel the disease process or is just an innocent bystander useful for measuring inflammation,” Szalai says. “But there is evidence suggesting it is directly involved in development of atherosclerosis and other risk factors for heart disease.” Many factors—some of them lifestyle choices people can modify—can cause inflammation and a rise in CRP, including smoking, obesity, and high blood pressure. But there are factors influencing CRP levels that people can’t change, particularly those written in their unique DNA. “Inheritable genetic variations in the CRP genes carried by individuals means some people have naturally higher levels of CRP,” says Szalai. “Higher baseline levels could indicate a higher-than-average risk for heart disease. Screening for genetic variations is a potential tool for placing people into high- versus low-risk groups or selecting those who might benefit from a drug targeting CRP.” [Learn more about Szalai's CRP research in this Web-only feature.] Redeeming Good CholesterolResearchers are vigorously searching for new ways to specifically target CRP and other factors involved in inflammation, but they have been stymied so far. “The drugs we already have that effectively treat cardiovascular disease, like statins and ACE inhibitors, clearly also decrease inflammation,” says cardiologist Louis Dell’Italia, M.D., who is the director of UAB’s Center for Heart Failure Research. “But every time we try to target one single aspect of inflammation with a specific drug, it hasn’t been beneficial.”
One promising avenue of research leads to HDL, the “good” cholesterol transporter. “HDL inhibits atherosclerosis, inhibits inflammatory response, inhibits bacterial inflammation,” says G.M. Anantharamaiah, Ph.D., an investigator in UAB’s Atherosclerosis Research Unit (ARU). “Studies also show that it inhibits Alzheimer’s inflammation, reduces kidney inflammation, and helps with diabetes.” Epidemiological studies show that “each one-milligram increase in HDL brings a 2 or 3 percent reduction in heart disease,” he adds. But simply boosting HDL levels doesn’t seem to be the answer, as demonstrated by the failure a few years ago of torcetrapib, a drug being tested by pharmaceutical giant Pfizer. Although torcetrapib increased HDL by 60 percent or more in patients, there were more deaths among trial participants who received the drug compared to those who received a placebo. What has become apparent is that HDL isn’t always good, says David Garber, Ph.D., another ARU investigator who is currently focusing on HDL and inflammation in diabetes. “In a normal individual, HDL is anti-inflammatory,” he says. “Recent research, however, shows that in many patients with diabetes and arthritis, their HDL is actually pro-inflammatory, and these are indeed people who have a higher risk for atherosclerosis.” The problem is that “HDL is actually a soup of different kinds of particles,” Garber says. “If certain proteins are either modified or removed, it becomes pro-inflammatory.” In effect, this means there is good HDL and bad HDL, he explains. Anantharamaiah, Garber, and other ARU investigators are refining two potent—and potentially revolutionary—drugs that may be able to induce pro-inflammatory HDL to switch back to its normal, anti-inflammatory state. The drugs mimic the key HDL components apolipoproteinA-I and apolipoprotein E, respectively. The apo-E drug, when tested in animal models, dramatically lowers LDL cholesterol levels and also combats inflammation. The apo-A-I drug, known as Oral HDL, is much farther along the path to clinical use: In preclinical testing it has shown a remarkable ability to reduce both inflammation and the size of atherosclerotic plaques. Interestingly, it does this even though it doesn’t appear to boost HDL levels. “There is no change in cholesterol level after administration, but yet the inflammation is inhibited,” says Anantharamaiah. “It’s not letting the lipoproteins enter into the walls of the blood vessels and get deposited.” “It’s still hard to tease out exactly what’s going on,” Garber adds. “The common mechanism seems to be improvement of HDL quality—as demonstrated in Phase I clinical studies in patients with established cardiovascular disease—and inhibition of inflammatory processes,” possibly because Oral HDL raises levels of superoxide dismutase. This essential enzyme dismantles the dangerous free radical superoxide, which is part of the inflammatory arsenal the body uses to dispatch invaders and its own damaged cells. To provide a long-term solution to atherosclerosis, Garber says, “you’ve got to go after the underlying problem, and we think Oral HDL is doing exactly that.”
|
 DESPITE THE PHENOMENAL SUCCESS of drugs like ACE inhibitors, beta blockers, and especially the cholesterol-lowering statins, heart disease is still the leading cause of death in the United States. For several years, investigators have had a new target in their crosshairs, however: inflammation.
DESPITE THE PHENOMENAL SUCCESS of drugs like ACE inhibitors, beta blockers, and especially the cholesterol-lowering statins, heart disease is still the leading cause of death in the United States. For several years, investigators have had a new target in their crosshairs, however: inflammation. Smoke Screen
Smoke Screen