Next Step for Regenerative Medicine
By Charles Goldthwaite Jr., Ph.D.
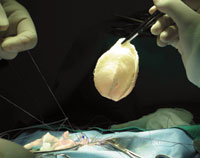 Surgeons at UAB successfully used Tengion’s Neo-Bladder™ to treat a common complication of spina bifida.
Surgeons at UAB successfully used Tengion’s Neo-Bladder™ to treat a common complication of spina bifida.
The idea of “growing” skin, bones, teeth, and blood vessels from cultured cells—and then implanting them into patients—was once the stuff of science fiction. But now that researchers have accomplished these feats, the field of regenerative medicine has set its sights on engineering entire new replacement organs that may one day offer a cure for everything from diabetes to heart disease. Such advances are made in increments, however, and the initial breakthroughs might well come from work on a very humble organ indeed: the bladder.
“Although many people take it for granted, the process of voiding urine is complicated,” observes pediatric urologic surgeon David Joseph, M.D. This is especially true for children with spina bifida, a developmental birth defect that occurs when openings form in a baby’s spine during pregnancy. These openings create problems in nerve transmission, and 95 percent of spina bifida patients develop a condition known as neurogenic bladder, in which disrupted nerve pathways lead to urinary incontinence, urinary tract infections, decreased bladder capacity, and often kidney disease.
Existing procedures treat the condition by surgically increasing the size of the bladder, but this carries substantial risks. So Joseph and colleagues at UAB were eager to take part in a phase-2 multisite clinical trial of a creative new approach: the Neo-Bladder. Designed by Tengion Corporation, this synthetic implant consists of a biodegradable scaffold that is covered with urothelial and smooth muscle cells cultivated from the patient. The Neo-Bladder is then sutured directly onto the patient’s bladder. Within weeks of implantation, the scaffold dissolves, leaving grafted tissue that is very similar to the patient’s native tissue.
“This approach expands the bladder’s capacity while avoiding some of the complications associated with surgical methods such as augmentation cystoplasty, in which a segment of the patient’s bowel is folded into a patch and attached to the bladder,” notes Joseph, who is a principal investigator for the ongoing phase-2 pediatric portion of the trial. “Although we are still assessing data, we have had no major safety concerns with the procedure. If we can eliminate the side effects of augmentation procedures, then the potential benefits to the patient are outstanding.”
UAB researchers and colleagues at Wake Forest University, Children’s Hospital Los Angeles, and Children’s Hospital Boston are now collecting data on the performance of the new implants. “With these types of procedures, patients and their families often report subjective benefits,” Joseph explains. “But to prove that this approach is successful, we must generate objective data.” Even as they await these results, however, the researchers have begun to develop a larger-scale trial that could begin as early as this year.
Joseph observes that this is only the latest chapter in the rich history of bladder regeneration approaches. And in the past, pioneering work in the bladder has paid dividends for other organs as well. “The bladder was the first organ used for regrowth procedures,” says Joseph. “If we can show that this approach works for the bladder, there is great potential for applying similar strategies to other organs whose tissue is damaged. We are really at the tip of the iceberg here.”