 Professor and Departmental Wellness Champion
Professor and Departmental Wellness Champion
Research Areas
Biosynthesis of retinoic acid
Biography
Natalia Y. Kedishvili received her M.S. degree in Biochemistry in 1982, and a Ph.D. degree in Biochemistry in 1987 from the Lomonosov's Moscow State University, Russia. After completing her postdoctoral fellowship at Indiana University School of Medicine, she was recruited by the University of Missouri-Kansas City, where she developed a research program in retinoid and steroid metabolism. Dr. Kedishvili joined the UAB faculty in 2004.
Research Interests
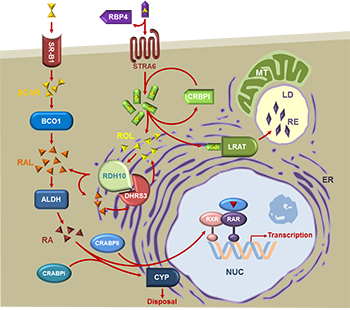 The work in my lab is focused on the enzymes and the mechanisms that control the biosynthesis of the bioactive derivative of vitamin A, retinoic acid [1]. Retinoic acid is a powerful small lipid molecule that exerts genomic actions through binding and activating nuclear transcription factors, retinoic acid receptors (RARs) [2], and non-genomic actions through binding to mRNAs in the cytoplasm [3], and activation of kinases via cellular retinoic acid binding protein type 1 [4]. Either too much or too little of retinoic acid is equally harmful; hence, the levels of retinoic acid in the cells are tightly controlled. The mechanisms that control the biosynthesis and degradation of retinoic acid are not yet fully understood. The research conducted in our laboratory uncovered the existence of a multisubunit protein complex that controls the baseline levels of retinoic acid in many cells. This complex is composed of at least two subunits of retinol dehydrogenase 10 (RDH10) and 2 or more subunits of dehydrogenase reductase 3 (DHRS3). Together, these two proteins form an oligomeric retinoid oxidoreductase complex (ROC) that regulates the rate of retinoic acid biosynthesis [5]. As we are progressing towards a better understanding of the fundamental mechanisms that control retinoic acid levels, new important and intriguing questions arise about the structure and regulation of the ROC; about the existence of other retinol dehydrogenases that may function in a cell-specific manner; about the mechanisms that disrupt the retinoic acid homeostasis, leading to disease; and about potential therapeutic interventions aimed to restore the homeostasis. This work has been continuously supported by the National Institute on Alcohol and Alcoholism for over 20 years.
The work in my lab is focused on the enzymes and the mechanisms that control the biosynthesis of the bioactive derivative of vitamin A, retinoic acid [1]. Retinoic acid is a powerful small lipid molecule that exerts genomic actions through binding and activating nuclear transcription factors, retinoic acid receptors (RARs) [2], and non-genomic actions through binding to mRNAs in the cytoplasm [3], and activation of kinases via cellular retinoic acid binding protein type 1 [4]. Either too much or too little of retinoic acid is equally harmful; hence, the levels of retinoic acid in the cells are tightly controlled. The mechanisms that control the biosynthesis and degradation of retinoic acid are not yet fully understood. The research conducted in our laboratory uncovered the existence of a multisubunit protein complex that controls the baseline levels of retinoic acid in many cells. This complex is composed of at least two subunits of retinol dehydrogenase 10 (RDH10) and 2 or more subunits of dehydrogenase reductase 3 (DHRS3). Together, these two proteins form an oligomeric retinoid oxidoreductase complex (ROC) that regulates the rate of retinoic acid biosynthesis [5]. As we are progressing towards a better understanding of the fundamental mechanisms that control retinoic acid levels, new important and intriguing questions arise about the structure and regulation of the ROC; about the existence of other retinol dehydrogenases that may function in a cell-specific manner; about the mechanisms that disrupt the retinoic acid homeostasis, leading to disease; and about potential therapeutic interventions aimed to restore the homeostasis. This work has been continuously supported by the National Institute on Alcohol and Alcoholism for over 20 years.
In addition to our basic science research, we are interested in identifying novel chemopreventive agents, rexinoids, which bind to retinoid X receptors (RXR) in the nucleus and inhibit carcinogenesis in UV-exposed skin. Our preliminary data suggest that a rexinoid developed at UAB, UAB30, acts by increasing the endogenous levels of anticarcinogenic retinoic acid in human and mouse skin [6]. This work is conducted in collaboration with Drs. Muccio, Athar, Elmets and Atigadda, and has been supported by a pilot project from the Comprehensive Cancer Center at UAB.
We are also interested in understanding the pathophysiology of mutations in photoreceptor-specific retinol dehydrogenase 12 (RDH12) [7]. Mutations in RDH12 lead to very early onset macular degeneration and photoreceptor cells death. However, the mechanism of this disease is not understood, which impedes the search for appropriate treatments. This work is conducted in collaboration with Drs. Steve Aller, Olga Belyaeva, and Marina Gorbatyuk, and has been supported by a pilot project from UAB Vision Science Research Center.
References
1. Kedishvili NY. Retinoic Acid Synthesis and Degradation. Subcell Biochem. 2016;81:127-161.
2. Rochette-Egly C. Retinoic acid signaling and mouse embryonic stem cell differentiation: Cross talk between genomic and non-genomic effects of RA. Biochim Biophys Acta. 2015 Jan;1851(1):66-75.
3. Chen N, Onisko B, Napoli JL.The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008 Jul 25;283(30):20841-7.
4. Wei LN.Cellular Retinoic Acid Binding Proteins: Genomic and Non-genomic Functions and their Regulation. Subcell Biochem. 2016;81:163-178.
5. Belyaeva OV, Adams MK, Wu L, Kedishvili NY. The antagonistically bifunctional retinoid oxidoreductase complex is required for maintenance of all-trans-retinoic acid homeostasis. J Biol Chem. 2017 Apr 7;292(14):5884-5897.
6. Wu L, Chaudhary SC, Atigadda VR, Belyaeva OV, Harville SR, Elmets CA, Muccio DD, Athar M, Kedishvili NY. Retinoid X Receptor Agonists Upregulate Genes Responsible for the Biosynthesis of All-Trans-Retinoic Acid in Human Epidermis. PLoS One. 2016 Apr 14;11(4):e0153556.
7. Lee SA, Belyaeva OV, Popov IK, Kedishvili NY. Overproduction of bioactive retinoic acid in cells expressing disease-associated mutants of retinol dehydrogenase 12. J Biol Chem. 2007 Dec 7;282(49):35621-8.
Education
Graduate School
Ph.D., Department of Biochemistry, School of Biological Sciences, Lomonosov Moscow State University, Moscow
Postdoctoral Fellowship
Indiana University School of Medicine
Contact
Office
Kaul Human Genetics Building
Room 440B
720 20th Street South
Birmingham, AL 35294-0024
Phone
(205) 532-3738
Email
nkedishvili@uab.edu