Professor and Vice Chair of Faculty Development
Research Areas
IgA nephropathy and the analysis of protein glycosylation in diseases by mass spectrometry
Research Interests
Our group makes use of high resolution Fourier transform mass spectrometry (FT-MS) to analyze the clustered O-glycans found on the immunoglobulin IgA1 as related to the pathogenesis of IgA nephropathy. This includes the site-specific localization and characterization of individual O-glycan chains in serum samples from patients with IgA nephropathy, enzymatic studies of glycosyltransferases, and many other protein biochemistry experiments. Our goal is to identify the aberrant IgA1 O-glycosylation pattern that leads to the mesangial deposits of IgA1-containing immune complexes as well as characterizing the other proteins involved in the pathogenesis of the disease. This may lead to alternative methods for diagnosing and monitoring the disease as well as identifying targets for therapeutic intervention.
 Glycosylation is one of the most common post-translational modifications of proteins. It is estimated that over half of mammalian proteins are glycosylated. Glycoproteins are vital for a wide range of biological processes including: transporting molecules, production of enzymes, acting as cell attachment-recognition sites, etc. The varying types and structures of glycoproteins allow them to adapt to these diverse functions. There are two types of protein glycosylation, O-linked and N-linked.
Glycosylation is one of the most common post-translational modifications of proteins. It is estimated that over half of mammalian proteins are glycosylated. Glycoproteins are vital for a wide range of biological processes including: transporting molecules, production of enzymes, acting as cell attachment-recognition sites, etc. The varying types and structures of glycoproteins allow them to adapt to these diverse functions. There are two types of protein glycosylation, O-linked and N-linked.
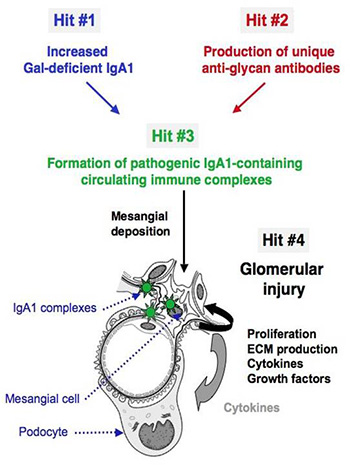
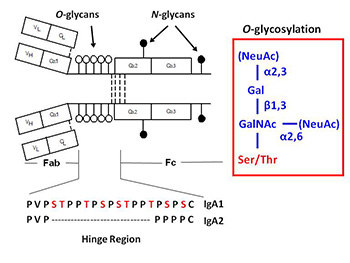 A variety of proteins are postranslationally modified with clustered sites of O-glycosylation. These are serine and threonine rich stretches within the amino acid sequence that have short O-glycan chains. (The picture to the left shows a stretch of clustered O-glyans on IgA1 hinge region.) Some examples of O-glycosylation include the immunoglobulin A (A1 isotype), mucins, and bacterial cell surface proteins. IgA nephropathy (IgAN, also known as Berger's disease) is the most common primary glomerulonephritis worldwide, with about 20-40% of patients developing end-stage renal failure. It is characterized by mesangial deposits of IgA1-
A variety of proteins are postranslationally modified with clustered sites of O-glycosylation. These are serine and threonine rich stretches within the amino acid sequence that have short O-glycan chains. (The picture to the left shows a stretch of clustered O-glyans on IgA1 hinge region.) Some examples of O-glycosylation include the immunoglobulin A (A1 isotype), mucins, and bacterial cell surface proteins. IgA nephropathy (IgAN, also known as Berger's disease) is the most common primary glomerulonephritis worldwide, with about 20-40% of patients developing end-stage renal failure. It is characterized by mesangial deposits of IgA1-
containing immune complexes. The carbohydrate side chains of IgA1 molecules play a pivotal role in the pathogenesis of IgAN. IgA1 contains a hinge region between the first and second heavy chain constant region domains with a high content of proline, serine, and threonine and usually have three to six O-linked glycan chains. IgAN is an autoimmune disorder that exhibit abnormal glycosylation of serum immunoglobulins (See IgAN). For a given protein with sites of clustered O-glycans, the protein isolated from a single source produces a population of variably O-glycosylated isoforms that we can observe by mass spectrometry. These isoforms usually show a distinct distribution of microheterogeneity in terms of number of chains, the sites of attachment and O-glycan composition at a given amino acid. Characterizing these clustered sites and understanding how thedistributions change under different biological conditions or disease states is an analytical challenge that our lab is tackling.
Education
Graduate School
Ph.D., University of Georgia, Athens, GA
Postdoctoral Fellowship
Florida State University
Contact
Office
Kaul Human Genetics Building
Kaul 524
720 20th Street S.
Birmingham, AL 35294-0024
Phone
(205) 996-4681
Email
renfrow@uab.edu