The United States Food and Drug Administration has announced its approval of the first genetic therapy treatment for sickle cell disease. The drug exagamglogene autotemcel, commercially know as Exa-cel, is a gene-editing therapy that can improve the quality of life for those living with SCD.
Julie Kanter, M.D., director of the University of Alabama at Birmingham’s Adult Sickle Cell Clinic and co-director of the Lifespan Comprehensive Sickle Cell Disease Program, explains what gene therapy is, how it works and what the next steps are in treatments for SCD.
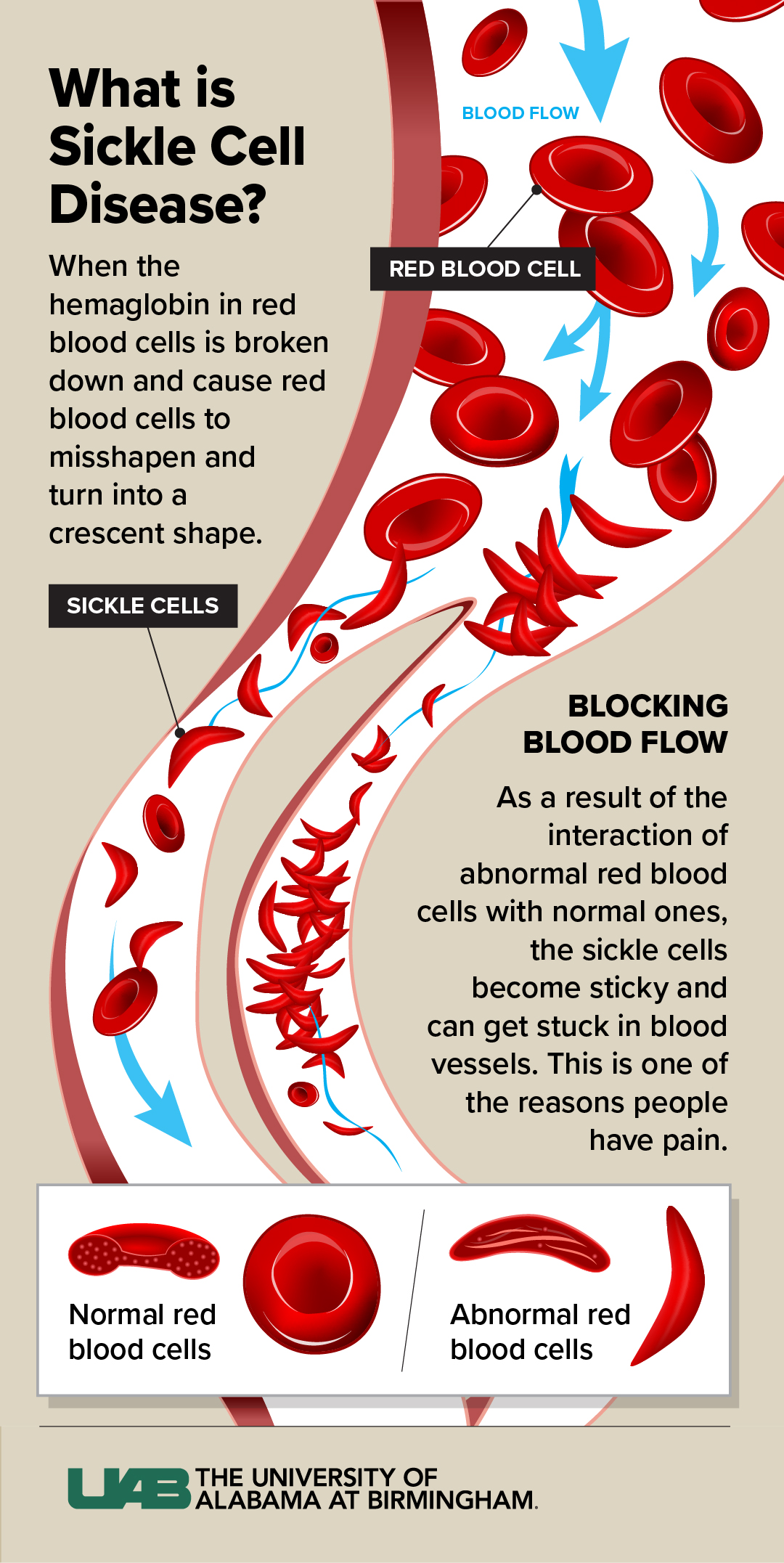
(Click to enlarge) Sickle cell disease is the most common and clinically significant inherited blood disorder across the nation, and now there is an FDA-approved gene therapy to help those living with SCD. Graphic by: Jody PotterWhat is sickle cell disease?
Although considered a rare disease, with fewer than 200,000 cases in the United States, SCD is the most common and clinically significant inherited blood disorder across the nation.
Sickle cell disease is a hemoglobin disorder. Hemoglobin is the protein inside red blood cells that transports oxygen from the lungs to the rest of the body. The hemoglobin of a person with SCD is abnormal and causes the red blood cells to misshape and form into a crescent, as opposed to the normal disc shape. As a result of the interaction between the red cell membrane and the hemoglobin, the red blood cells become sticky, which causes the cells to get wedged inside tiny blood vessels in people with sickle cell disease.
“Normally, red blood cells flow through like water through pipes. When the cells get dehydrated or lose their oxygen, they can get stuck in a person’s ‘tiny pipes,’” said Kanter, who is also a professor in the UAB Division of Hematology and Oncology. “This blockage keeps the red blood cells from getting to their destination and is a main reason people have pain or organ damage with SCD.”
SCD occurs in about one out of every 365 Black or African American births, according to the Centers for Disease Control and Prevention, and about one in 13 Black or African American babies is born with the sickle cell trait.
What is gene therapy?
SCD is caused by a single gene mutation that results in the abnormal hemoglobin; gene therapy is designed to change other parts of the DNA and help the patient create new healthy hemoglobin.
“The current gene therapies available do not target the sickle mutation, so the patient will continue to make sickle hemoglobin,” Kanter said. “However, the goal is the therapy will help them make enough healthy hemoglobin that it will prevent the red blood cells from sickling or becoming sticky.”
The newly approved drug targets a gene that controls the production of fetal hemoglobin during the first year of life. After a baby is born, the fetal hemoglobin gene is turned off, and the body starts producing adult hemoglobin. Exa-cel edits the off-switch and “turns it back on” so that the individual will begin producing fetal hemoglobin once again, and in large amounts. This process will help the individual with SCD as the fetal hemoglobin protects red blood cells and can help prevent the cell from sickling, most of the time.
“It is important that we view gene therapy, for now, as transformative therapy not a cure,” Kanter said. “When you think about a cure, that should mean that you no longer experience issues due to the disease — we do not have the data yet to know this of the current gene therapies. We do not know if organs are completely protected and if treated individuals will no longer be at risk of other complications that are caused by SCD. Right now, we know the therapy dramatically reduces vaso-occlusive pain crisis and improves quality of life, which is a great start.”
Read about the gene therapy research Kanter had published in the New England Journal of Medicine here.
What are the next steps?
Kanter says the FDA approval brings a lot of hope to all those living with SCD, but it could be an extended period before sickle cell centers are able to offer the therapy to all patients. She explains that this makes continued research and clinical trials even more important.
“This is not a ‘one and done,’” Kanter said. “I hope the current approval continues to spur even more improvements, new therapies and new developments to create hope and a better life for people with sickle cell disease.”
Kanter is looking ahead to more gene therapies’ receiving FDA approval and seeing the lives of her patients in the UAB Adult Sickle Cell Clinic be transformed.