Faculty active in this area of research are listed below. For a brief description of their research interests, click on their name in the list. Clicking on the name at the beginning of the brief description links to their detailed personal website.
Katie Alexander, PhD H. pylori infection is firmly established as a risk factor for the development of gastric adenocarcinoma, but relatively little is known about how its colonization of the stomach contributes to epithelial dysfunction, particularly at the stem cell level. My current research project uses stem cell organogenesis to examine H. pylori infection in the human stomach and determine whether infection upregulates epithelial ST6Gal-I expression, a glycosyltransferase that is overexpressed in multiple mucosal cancers including ovarian, pancreatic and colonic. In another project, I have uncovered a dysregulation of ST6Gal-I expression in Barrett’s esophagus, a pre-cancerous lesion that is associated with an increased risk of developing esophageal adenocarcinoma. Furthermore, I also am interested in the epigenetic regulation of mucosal cancers.
William W. Andrews, MD Dr. Andrews is a Professor in the UAB Maternal-Fetal Medicine Division, and has been the Director of the Obstetrics and Gynecology Infectious Disease Research Laboratory since its creation in 1990. Dr. Andrews' research interests relate to obstetrical infections and infection-related preterm birth. He is the PI for an investigation of Interconceptional Antibiotics to Prevent Recurrent Preterm Birth that was recently completed and is the protocol chairman of a prospective randomized trial within the NICHD MFM Units Network of antibiotics to prevent preterm birth in women with elevated mid-trimester cervical fibronectin that is in the analysis phase. He is also the PI of Project IV within the UAB Rural Perinatal Emphasis Research Center which is an evaluation of a neonatal sepsis-like syndrome related to in utero maternal cytokine exposure. Most recently he was awarded a contract to perform "A Longitudinal Study of Bacterial Vaginosis". Dr. Andrews is a consultant and co-investigator on numerous other interdisciplinary projects related to genital tract infections, sexually transmitted diseases, and preterm birth, and he is a co-investigator within the International Clinical Epidemiology Network (INCLEN).
T. Prescott Atkinson, MD, PhD Research in my laboratory is focused on the role of infection in chronic diseases, especially arthritis and asthma. Ongoing projects in coordination with the UAB Diagnostic Mycoplasma Laboratory are designed to identify mycoplasmas and ureaplasmas in human samples with particular emphasis on the role of those organisms in chronic asthma and extreme prematurity respectively. Previous studies in my laboratory established that Mycoplasma pneumoniae is able to activate mast cells to produce IL-4 through sialic acid-dependent binding to the high affinity receptor for IgE, a finding with potential implications in the pathogenesis of asthma and potentially a general mechanism in the activation of cells of the immune system by that organism. Work is currently proceeding to determine the current prevalence of macrolide resistance strains of M. pneumoniae in the Birmingham area. I am also actively engaged in the development of rational strategies to determine the molecular basis for unidentified immunodeficiencies in patients in my weekly clinical immunology clinics at Children’s of Alabama. Such patients may represent natural “knockouts” or dominant negative mutations in signaling molecules and provide valuable insights into critical steps in receptor signaling in the human immune system.
.
Khurram Bashir, MD Clinical, epidemiological and outcomes research in immunologic disorders of the central nervous system, specifically multiple sclerosis
.
Anju Bansal, PhD My research interests have largely centered on examining the contribution of adaptive T-cell responses in context of infection and vaccination. Specifically, my research has focused on understanding the role of T cells in HIV-1 transmission, HIV-1 pathogenesis and candidate HIV-1 vaccines in relation to host genetics. This information is imperative to the future development of an efficacious HIV vaccine. I am also interested in determining the role of dysfunctional T cells in non-AIDS defining cancers (NADC), especially those that are caused by oncogenic viruses among HIV infected individuals.
Suresh Boppana, MD Dr. Boppana’s laboratory is studying the pathogenesis of congenital cytomegalovirus (CMV) infections. Congenital CMV infection is the most frequent congenital infection and a leading cause of brain damage and sensorineural hearing loss in children. The focus of the work in our laboratory include: 1. Understanding the intrauterine transmission of CMV in women who were CMV seropositive before pregnancy. We recently showed that acquisition of a new CMV strain is associated with intrauterine transmission and damaging congenital infection in immune mothers. Our ongoing studies will attempt to define the role of maternal reinfection, maternal strain-specific immune responses and other factors in transplacental transmission and damaging congenital infections in infants born to immune mothers; 2. Definition of the mechanisms of hearing loss, in particular, the development of late onset and/or progressive hearing loss in children with congenital CMV infection. We are investigating the role of virus burden and characterizing the virus specific cellular immune responses in congenitally infected children to better delineate the pathogenesis of hearing loss in children with congenital CMV infection; 3. Understanding the virologic and immunologic characteristics of primary CMV infection.
Donald J. Buchsbaum, PhD. Dr. Buchsbaum’s research is focused on the use of agonistic monoclonal antibodies that bind to the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors for cancer therapy in combination with chemotherapy agents or radiation. The research involves investigation of the mechanisms of enhanced cytotoxicity produced by combination treatment with TRAIL death receptor antibodies and chemotherapy or radiation therapy. Orthotopic and metastatic breast, ovarian, and pancreatic cancer xenograft models are being used to optimize therapeutic regimens. In collaboration with investigators from the Divisions of Gynecologic Oncology and Clinical Immunology, surgical specimens from patients with ovarian cancer are being utilized to develop a tumor slice assay to attempt to correlate in vitro cytotoxicity sensitivity to treatment with death receptor antibody and chemotherapy to patient survival following treatment with humanized antibody and taxol/carboplatin. If successful, this could be used as a predictive assay to select patients for treatment. We are also investigating the use of small molecule modulators of apoptosis to increase the level of tumor cell killing. Other research involves investigating the mechanism by which basal-like triple negative breast cancers are sensitive to death receptor antibody treatment, and the response of basal-type breast cancer stem cells to treatment with TRAIL receptor antibodies, gamma secretase inhibitors, and chemotherapy.
R. Pat Bucy, MD, PhD Dr. Bucy is interested in the regulation of immune responses by T cells, particularly the forms of regulation that develop in vivo in situations with chronic antigen presence. Conventional experimental systems have used model antigens given in discreet inoculations so that the clearance of antigen is the dominant overall control mechanism. In physiological situations such as solid organ transplantation, chronic viral diseases, and organ specific autoimmune diseases, antigen is usually not cleared, but the immune system develops various control mechanisms that limit immune damage. In addition to his role as the Director of the UAB Medical Scientist Training Program (joint MD/PhD training), Dr. Bucy's lab is engaged in a wide range of projects with a translational focus, that span the gamut of basic mechanistic studies in mice to active design of human clinical trials. Active current projects include use of TCR transgenic mice to study murine heart transplant tolerance, analysis of T cell population dynamics in response to various forms of immunization, studies of viral and cellular dynamics in SHIV infected Rhesus Macaques, and a substantial series of studies focused on therapeutic immunization of HIV infected people and assessment of changes in immune function in these people. In all of these systems, multiple techniques are used including flow cytometry, immunohistochemistry, cell culture techniques, production of novel transgenic mice, real-time RT-PCR,. and in situ hybridization analysis of viral and cellular RNA species.
Noel K. Childers, DDS, PhD Dr. Childer's current studies involve investigations aimed at identifying safe and effective mucosal immunization delivery systems. Specifically, studies examine the characteristics of liposomes that are important in potentiating immune responses to orally or nasally administered S. mutans antigens. This involves in vitro studies of the physical characteristics of liposomal antigen preparations as well as in vivo studies into the uptake and processing of liposome preparations in rats. Following animals studies of the efficacy of liposomal S. mutans antigen vaccines, studies have been initiated for human FDA Phase I clinical trials studying the safety and immunogenicity of liposomal oral and nasal immunization. The overall goal of these studies are to identify a safe and effective oral immunization strategy which is protective against dental caries.
Clinical research interests also include studies to determine risk factors for oral complications in children with cancer and HIV infection. These studies have assessed various clinical and immunological factors involved in the development of oral lesions in medically compromised children. The goal of this research is to develop and test protocols that will prevent the occurrence or severity of oral complications in children identified to be at risk. Additionally, research efforts have involved assessment of the prevalence of dental disease in children as related to access to care. Related studies have assessed the effectiveness of dental sealants in prevention of dental caries using Medicaid claims as well as Jefferson County Department of Public Health records.
Randy Q. Cron, MD, PhD Host transcription factors exploited by HIV-1. HIV-1, the cause of AIDS, has infected over 40 million individuals world-wide. Although vast improvements in therapy have been developed over the last decade, HIV-1 cannot be totally eliminated from the host due to its ability to enter a resting or latent state in 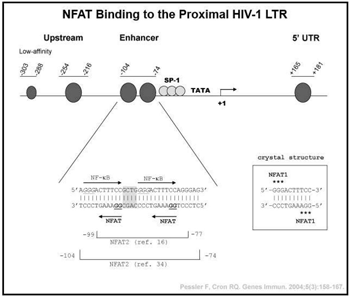 CD4 T cells. Because HIV-1 relies on host transcription factors to replicate, we are exploring the role of the calcium activated nuclear factor of activated T cells (NFAT) transcription factors in regulating HIV-1 transcription. We and others have shown that the CsA-sensitive NFAT proteins bind to the proximal HIV-1 promoter/long terminal repeat (LTR) in vitro and up-regulate HIV-1 transcription. We have further demonstrated that NFAT proteins bind to the integrated HIV-1 LTR in primary human CD4 T cells in vivo by chromatin immunoprecipitation, and this binding is disrupted by the regulatory T cell transcription factor, FOXP3. In addition, we are attempting to exploit NFAT activation as a means of activating HIV-1 LTR activity in latently infected cells. Recently, we identified a novel binding site for the c-maf transcription factor located adjacent to the proximal NFAT sites in the HIV-1 LTR. Our studies reveal synergistic transcriptional activation and increased infection of HIV-1 by c-maf, NFAT2, and NFΚB p65 in primary human IL-4-producing CD4 T cells. Thus, c-maf will likely be a novel therapeutic target in the treatment of HIV-1.
CD4 T cells. Because HIV-1 relies on host transcription factors to replicate, we are exploring the role of the calcium activated nuclear factor of activated T cells (NFAT) transcription factors in regulating HIV-1 transcription. We and others have shown that the CsA-sensitive NFAT proteins bind to the proximal HIV-1 promoter/long terminal repeat (LTR) in vitro and up-regulate HIV-1 transcription. We have further demonstrated that NFAT proteins bind to the integrated HIV-1 LTR in primary human CD4 T cells in vivo by chromatin immunoprecipitation, and this binding is disrupted by the regulatory T cell transcription factor, FOXP3. In addition, we are attempting to exploit NFAT activation as a means of activating HIV-1 LTR activity in latently infected cells. Recently, we identified a novel binding site for the c-maf transcription factor located adjacent to the proximal NFAT sites in the HIV-1 LTR. Our studies reveal synergistic transcriptional activation and increased infection of HIV-1 by c-maf, NFAT2, and NFΚB p65 in primary human IL-4-producing CD4 T cells. Thus, c-maf will likely be a novel therapeutic target in the treatment of HIV-1.
Genetic defects in lymphocyte cytolysis in macrophage activation syndrome. Macrophage activation syndrome (MAS) is a hyper-inflammatory immune response in children and adults that is often triggered by certain infectious (e.g. EBV), autoimmune (e.g. lupus), autoinflammatory (e.g. Still disease), and oncologic (e.g. T cell leukemia) disorders. MAS results in pro-inflammatory cytokine storm leading to pancytopenia, coagulopathy, central nervous system dysfunction, and multi-organ system failure. MAS is frequently lethal like its cousin disease familial hemophagocytic lymphohistiocytosis (fHLH). fHLH is uniformly fatal if not treated aggressively and typically presents in the first few months of life in infants 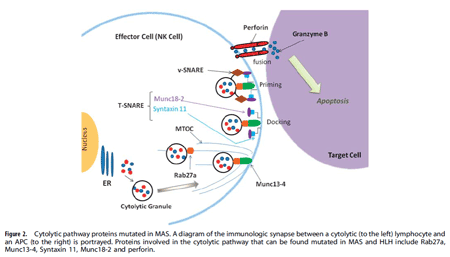 with bi-allelic genetic defects in one of the proteins involved in perforin mediated cytolysis by natural killer (NK) cells and CD8 cytotoxic lymphocytes. Recently, mono-allelic (heterozygous) mutations in cytolytic pathway proteins (e.g. perforin, Munc13-4, Rab27a, etc.) have been identified in a substantial percentage of MAS patients presenting beyond infancy. In our MAS patient cohort, we have identified several mutations, including novel mutants, in a variety of cytolytic pathway genes. Using lentiviral transduction of mutant and wild-type genes into NK cells, we demonstrate decreased cytolytic activity by over-expression of the mutant genes, suggesting a partial dominant-negative effect. These studies suggest that there are likely genetic predispositions to develop MAS, and we are currently exploring the novel mutations and their pathophysiological consequences on lymphocyte mediated cytolytic function.
with bi-allelic genetic defects in one of the proteins involved in perforin mediated cytolysis by natural killer (NK) cells and CD8 cytotoxic lymphocytes. Recently, mono-allelic (heterozygous) mutations in cytolytic pathway proteins (e.g. perforin, Munc13-4, Rab27a, etc.) have been identified in a substantial percentage of MAS patients presenting beyond infancy. In our MAS patient cohort, we have identified several mutations, including novel mutants, in a variety of cytolytic pathway genes. Using lentiviral transduction of mutant and wild-type genes into NK cells, we demonstrate decreased cytolytic activity by over-expression of the mutant genes, suggesting a partial dominant-negative effect. These studies suggest that there are likely genetic predispositions to develop MAS, and we are currently exploring the novel mutations and their pathophysiological consequences on lymphocyte mediated cytolytic function.
Randall S. Davis, MD Dr. Davis’ laboratory investigates pathways of normal lymphocyte differentiation to determine the mechanisms that contribute to lymphomagenesis, autoimmunity and immunodeficiency. This work largely focuses on an ancient family of Ig-like receptors with tyrosine-based activating or inhibitory signaling potential. A search for possible Fc receptor relatives identified several novel Ig superfamily genes in humans and mice termed Fc receptor-like molecules (FcRL). FcRL family members are preferentially expressed in B lymphocytes and differentially identified in B lineage malignancies. Follow-up studies have identified eight human and seven mouse relatives in total. The recognition of this family has significant implications for understanding connections between innate and adaptive humoral immunity, the regulation and terminal differentiation of B cells into memory and plasma cells, and possibly, the pathogenesis of B cell malignancies and autoimmunity. Their expression patterns, signaling potential, ligands, and translational potential are areas of active investigation.
Jessy Deshane, PhD Dr. Deshane is committed to an academic career combining basic and translational research with an emphasis on inflammatory diseases of the airway. The focus of Dr. Deshane's research program is to enhance our understanding of the role of myeloid-derived regulatory cells in chronic airway inflammatory diseases. Asthma is a chronic inflammatory disease of the airways in which innate and adaptive immune cells participate as drivers of the inflammatory response. Free radical species have long been implicated as critical mediators of the asthmatic inflammatory process. Dr. Deshane's studies in a mouse model of allergic airway inflammation have established that subsets of free radical-producing myeloid-derived regulatory cells (MDRC) are master regulators of airway inflammation. They are potent modulators of both T cell responses and airway hyper-responsiveness. Dr. Deshane has identified human MDRC with similar function in bronchoalveolar lavage of asthmatics. Her current research interests are (1) to explore the free radical and cytokine/chemokine mediated mechanisms underlying the differentiation and function of myeloid derived regulatory cells in the establishment of airway inflammation and resolution of inflammation (2) to investigate MDRC- mediated regulation of the balance of Tregulatory cells and Th17 cells which control the tolerance vs inflammation (3) to understand how environmental pollutants such as tobacco smoke would impact MDRC function and contribute to exacerbation of inflammation in asthmatic smokers. These studies will provide insight into the role of MDRC in tobacco related pathology of the lung.
Steven R. Duncan, MD Research interests of Dr. Duncan center on parsing out the immunological mechanisms involved in the pathogenesis of some morbid and mostly refractory chronic lung diseases, particularly idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease. Ongoing studies include those to better understand the processes by which human T-cells undergo genomic, phenotypic, and functional changes after repeated antigen encounters, and explorations of ways to specifically target these cells or interfere with their functions. A recently developed human-chimeric mouse model, in which these animals are reconstituted with a human adaptive immune system will be helpful. Other investigations in progress include more detailed characterization of the autoantibody repertoires in these disease populations, with the aim of identifying autoantibody (and T-cell) specificities with greatest utility in diagnostic or prognostic assays. Additional, interrelated projects include further explorations of recently discovered mechanisms by which T-cells regulate fibroblast production of extracellular matrix, and high resolution sequencing (and functional studies) of novel immunogenetic regulatory polymorphisms that confer high risks of developing these chronic lung diseases. In addition to these bench studies, we plan to continue and extend early phase trials of novel immunological response modifiers in these patients.
Jeffrey C Edberg, MD Wegener’s granulomatosis, an anti-neutrophil cytoplasmic antibody (ANCA)-positive systemic small vessel vasculitide, is characterized by inflammatory lesions with granuloma formation in the upper and lower airways, pauci-immune glomerulonephritis, and anti-proteinase 3 autoantibodies. Although Wegener’s granulomatosis is considered idiopathic, there has been substantial interest in environmental factors as either etiologic or accelerating risk factors, with Staphylococcus aureus having attracted substantial attention as one such environmental factor. Although consensus about etiology remains elusive, the nature of the host response has emerged as an important determinant for disease phenotype and severity. Although Wegener’s granulomatosis occurs sporadically, and does not show classical familial clustering and transmission characteristic of some autoimmune diseases, the identification of important genetic factors in Wegener’s granulomatosis is not only feasible but also potentially very fruitful in providing insights into pathogenesis and potential therapeutic targets. Building on the clinical trial of etanercept in Wegener’s granulomatosis, Dr. Edberg, in collaboration with Drs. Kimberly and Kaslow, is developing a renewable genetic repository to explore the relationship between the Wegener’s granulomatosis diathesis and genetic polymorphisms in candidate molecules, selected for their role in pathophysiology. These studies also will involve identification of new polymorphisms in such molecules and application of these to this cohort.
Craig A. Elmets, MD Dr. Elmets’ research focuses on the interaction of environmental agents with the skin. His research has particular relevance to skin cancer and cutaneous allergic reactions. In the area of skin cancer, his interests are on skin cancer chemoprevention and therapy. He has played a key role in defining the mechanisms by which the immune system controls the development of skin cancers. More recent studies have centered on the identification of new agents that can protect against skin cancer and on the non-surgical treatment of these malignancies. These include the arthritis drug celecoxib and extracts of green tea. He has also played a major role in the development of photodynamic therapy as a treatment for cancer. Photodynamic therapy utilizes light activated drugs to eradicate cancer. Dr. Elmets’ other area of research is on allergic contact dermatitis, of which poison ivy is the best-known example. He is evaluating better and more accurate diagnostic techniques for contact allergies. His studies have shown that certain proteins called cytokines are synthesized in skin cells from allergic individuals exposed to contact allergens but not in those obtained from people who are the not allergic. These findings provide the conceptual framework for the development of a diagnostic test for skin allergy testing, which can used by physicians and by industry as an alternative to animal testing prior to the introduction of new products into the marketplace.
Charles O. Elson III, MD The central focus of the laboratory is on the regulation of mucosal immune responses, particularly the mucosal immune response to the microbiota, which represent the largest mass of antigen encountered by the immune system. The cellular and molecular mechanisms that maintain mucosal immune homeostasis are being defined. When these mechanisms fail, pathogenic effector T cells are generated that result in colitis. We have cloned a set of immunodominant antigens of the microbiota that stimulate such pathogenic T cells and result in inflammatory bowel disease. Among these cloned antigens, previously unknown bacterial flagellins have emerged as a major cluster. Seroreactivity to these flagellins is found in multiple experimental models of colitis in mice and in half of patients with Crohn's disease. These antigens drive a newly described CD4 T cell effector subset making IL-17 (Th17) which appears to be responsible for disease progression. A T cell receptor transgenic mouse reactive to CBir1 flagellin has been generated and is being used to study the innate and adaptive immune response to these microbiota antigens. A second major effort is in the identification of T reg cells in the intestine that recognize microbial antigens and maintain homeostasis. The mechanisms whereby such cells are induced are being defined and the application of these cells to prevent or treat intestinal inflammation is being tested. Lastly, a microbiota antigen microarray has been constructed which can be used to analyze serologic reactivity to the microbiota in both mouse and human. Sera from various human populations are presently being analyzed.
James F. George, PhD Dr. George’s research program is concentrated on the role of heme oxygenase-1 in modulation of immune responses with a particular emphasis on renal and cardiac injury as well as vascular disease in the context of kidney and cardiac disease after transplantation or ischemic injury. Innate immunity is a major factor in the response to acute kidney injury, both locally (within the kidney) and systemically. His group has shown that a major point of involvement of HO-1 in immune regulation is through its expression in cells of the mononuclear phagocyte system. HO-1 strongly affects the function and migration of cells involved in regulation of T cell responses and innate immunity. This work has involved detailed analyses of intrarenal leukocyte populations using flow cytometry, imaging, single cell RNAseq and spatial transcriptomics to determine lineage relationships, migration, and patterns of gene expression in a variety of newly developed and validated animal models of acute and chronic renal injury. In 2007, together with Dr. Anupam Agarwal, the Director of the Division of Nephrology, they established The UAB Small Animal Microsurgical Core Facility in order to provide investigators with the ability to perform high complexity surgical procedures in rodents, especially mice which are more difficult because of their small size. Dr. George has a strong commitment to graduate and post-graduate education. He has mentored a number of fellows and graduate students who now have outstanding careers in academic biomedical research. He has been recognized by the Dean of The Graduate School for Outstanding Mentorship at UAB.
Other Immungenetic Investigations. My colleagues and I have also studied host genetic factors involved in various other conditions. These include non-Hodgkin lymphoma, Kaposi sarcoma, varicella-zoster virus infection, Kawasaki syndrome, and response to other vaccines (e.g., HBV).
Paul Goepfert, MD Dr. Goepfert's research focuses on the impact of cross-reactive CD8 T cells on HIV-1 viral control and evolution, as well as on defining the biological relevance of HIV-1 adaptation to CD4 T Cell responses
Zdenek Hel, PhD Innate immune regulatory activity and neutrophil dysregulation as a driving mechanism of pathogenesis in HIV-1-infection. . Recent evidence demonstrates that neutrophils, the most abundant nucleated immune cell population in the body, play an important role in the regulation of adaptive and innate immune systems. We have shown that neutrophils from HIV-1-infected individuals display an activated phenotype, specific transcriptional profile, and increased rate of degranulation. We propose that HIV-1 infection is associated with altered myeloid cell homeostasis resulting in changes in the population frequency and functional activity of diverse granulocytic populations. Dysregulation of granulocytic recruitment, function, and clearance contributes to the pathogenesis of cardiovascular and liver diseases associated with HIV-1 infection. Specifically, neutrophils in the blood of HIV-1-infected individuals express high levels of PD-L1 that is induced by HIV-1 virions and products of microbial translocation including lipopolysaccharide (LPS). Neutrophil PD-L1 levels correlate with the expression of PD-1 on CD4+ and CD8+ T cells, elevated levels of neutrophil degranulation markers in plasma, and increased frequency of low density neutrophils expressing the phenotype of granulocytic myeloid-derived suppressor cells (G-MDSCs). Neutrophils purified from the blood of HIV-1-infected patients suppress T cell function via several mechanisms including PD-L1/PD-1 interaction and production of reactive oxygen species (ROS). The accumulated data suggest that chronic HIV-1 infection results in an induction of immunosuppressive activity of neutrophils characterized by high expression of PD-L1 and an inhibitory effect on T cell function. This newly identified mechanism of immune suppression mediated by neutrophils may alter our understanding of HIV-1 pathogenesis. Furthermore, we have shown that neutrophils from HIV-1-infected individuals display high capacity to undergo NETosis. Production of neutrophil extracellular traps (NETs) likely contributes to increased risk of cardiovascular and liver diseases in HIV-1-infected individuals.
Neutrophils and cancer. Our research focuses on neutrophils and granulocytic myeloid-derived suppressor cells (G-MDSCs), cell populations that have been recently identified to play a critical role in the regulation of adaptive and innate immune responses in cancer and chronic inflammatory conditions. Production of neutrophil extracellular traps (NETs) by neutrophils contributes to increased risk of cardiovascular and liver disease in cancer patients.
The impact of hormonal contraceptives on HIV-1 acquisition and transmission. Safe and effective methods of contraception represent a critical component of preventive health care reducing maternal and infant mortality, especially in women living in resource-limited settings. Depot medroxyprogesterone acetate (DMPA; Depo-Provera) is a highly effective progestin-based contraceptive and one of the most commonly used contraceptives in sub-Saharan Africa. Several epidemiological studies indicate an association between the use of DMPA and an increased risk of HIV-1 infection. Modelling studies indicate that the use of injectable contraceptives may be responsible for hundreds of thousands of new HIV-1 transmissions annually. It is therefore critically important to identify safe forms of contraception without a significant deleterious effect on systemic and mucosal immune environment. We demonstrated that medroxyprogesterone acetate (MPA) suppresses antigen- immune function of T cells and dendritic cells via direct and indirect mechanisms and increases the rate of HIV-1 proliferation. In a clinical study performed at UAB, we analyzed vaginal biopsies and various immune parameters in the blood of women using various forms of hormonal contraceptives. We showed that the use of MPA is associated with thinning of vaginal epithelial wall and decreased production of IFN-α by plasmacytoid dendritic cells. We have shown that MPA reduces defense mechanisms of genital epithelium by suppression of factors critical for the barrier function and structural integrity of the vaginal and cervical epithelium. Decreased production of these factors reduces the resistance of genital epithelial tissue to microabrasions and increases the probability of HIV-1 transcytosis and transmigration leading to an exposure of target cells in the parabasal epithelium and lamina propria. Furthermore, DMPA and NuvaRing (etonogestrel) significantly suppress the cervicovaginal levels of principal anti-HIV-1 inhibitory factors human β-defensin 2 and 3 and secretory leukocyte protease inhibitor (SLPI). In a recent randomized clinical study in Lusaka, Zambia, we showed that administration of MPA decreases the production of several factors in the cervicovaginal fluid of HIV-1-infected women that may contribute to higher shedding of the virus and potentially to increased rates of viral transmission. In search for safe contraceptives, we have demonstrated that norethisterone (NET) and levonorgestrel (LNG) do not inhibit the function of dendritic cells and T cells and therefore represent safe potential alternative to DMPA.
Robert P. Kimberly, MD Our laboratory is interested in the role of genetic factors in the normal function of the immune system and in development of autoimmune and immune-mediated inflammatory diseases such as systemic lupus erythematosus and systemic vasculitis. Our approach has focused on receptors for immunoglobulin (Fc receptors) as a model system and has explored molecular mechanisms of receptor signaling and the molecular basis for receptor polymorphisms in humans. Studies in cell lines and in normal donors have demonstrated that despite the common theme of receptor-induced tyrosine phosphorylation, the various human Fc receptors engage different signaling elements which are reflected in important distinctions in function. Similarly, allelic variations in receptor structure profoundly affect receptor function, and certain low-binding alleles are enriched in SLE patients. More active alleles are over-represented in patients with vasculitis and severe renal disease. Other genes and gene families are being pursued as they are identified as candidate genes through genome wide association studies. These genes include complement receptors, cytokine genes and their promoters, signal transduction molecules, and members of the TNF superfamily.
Sixto M. Leal, MD, PhD Utilizing model systems to identify key mediators of microbial detection and killing by phagocytic cells. Fungal Immunology and Mold pathogenesis. Prognostic markers and pathogenesis of SARS-CoV-2 infection. Development of novel molecular assays to more accurately diagnose infectious diseases.
Jiri Mestecky, MD, PhD Molecular-cellular interactions involved in the differentiation of B lymphocytes and epithelial cells of the mucosal immune system and the novel approaches for the induction of the humoral immune response in external secretions represent the primary area of interest in our laboratory. Regulation of the expression of immunoglobulin isotypes, with emphasis on IgA and J chain synthesis, are studied in human systems under normal as well as pathologic conditions. The interactions of IgA molecules of various properties (including those found in IgA-containing immune complexes) with lymphoid cells, macrophages, hepatocytes, eosinophils, and epithelial cells are investigated in a variety of human diseases afflicting the gastrointestinal and genitourinary tracts. Development of vaccines against AIDS and sexually transmitted diseases is pursued using novel antigen delivery systems and combination of immunization routes to stimulate mucosal immunity in the genital tract.
Tanecia Mitchell, PhD Dr. Mitchell’s scientific career has been devoted to studying the role of mitochondria and oxidative stress in kidney related disorders. Dr. Mitchell’s current research focuses on understanding the role of monocytes and macrophages during calcium oxalate kidney stone disease. In particular, her laboratory is investigating the importance of diet on immune cell metabolism and anti-bacterial response using experimental models and clinical trials.
John D. Mountz, MD, PhD A hallmark of autoimmune disease is the development of autoantibodies that can cause disease. My laboratory has identified that the second recombinant inbred strain of B6 x DBA/2 (BXD2) spontaneously produces very high levels of pathogenic autoantibodies. Single antibodies produced by hybridomas from spleens of these mice transfer arthritis or glomerulonephritis in normal mice. By 3 months of age, the spleens of BXD2 mice are greatly enlarged and are packed with numerous large, spontaneous germinal centers (GCs). This GC development is promoted by high levels of Th17 and IL-17 in these mice. IL-17 signals through the IL-17a receptor in B cells resulting in increased classical NF-κB pathway activation. This activates several genes, including regulators of G-protein signaling (RGS) 13 and 16. Upregulation of RGS genes impairs signaling through CXCR4/CXCL12 and CXCR5/CXCL13 to arrest migration and movement of T cells and B cells. This enables prolonged and stable interaction of B cells and CD4 T cells. Key ongoing questions in my laboratory include what is the mechanism for increased Th17 development. IL-6 is highly produced by B cells, macrophages and plasmacytoid dendritic cells (PDCs). TGF-β, however, is not greatly increased. What are the factors, in combination with IL-6, that promote high Th17 development in BXD2 mice? How does Th17 signal through B cells? Our recent evidence indicates that IL-17 signaling requires both TRAF6 and ACT1, which has been identified in IL-17 signaling pathways. Current ongoing work is to determine the mechanism of increased NF-κB signaling in response to IL-17 in B cells. Also using RGS13 KO and RGS16 KO mice, we wish to determine which of these RGS proteins is highly essential for development of spontaneous autoreactive GCs. We also wish to identify the most promising points for interruption of IL-17 signaling that upregulates RGS expression in B cells. Other studies include detailed analysis of the effect of IL-17 on B cell chemotaxis in response to CXCL12 and CXCL13. These include in vitro chemotactic chamber analysis, and live imaging analysis using confocal microscopy.
A second area of interest is the role of DR5 apoptosis in arthritis and autoimmune Disease. TRAIL-DR5 apoptosis signaling is very similar to FAS apoptosis signaling involving mitochondrial amplification loop and Bcl-2 family members, as well as direct induction of apoptosis through caspase activation resulting in terminal caspases 3, 5, and 7 activation. The TRAIL-DR5 apoptosis signaling pathway, like Fas, is inhibited by FLIP-L and XIAP (inhibitors of apoptosis proteins). DR5 is upregulated on synovial fibroblasts of patients with rheumatoid arthritis and in Collagen-II mouse model of arthritis. To determine mechanisms of DR5 apoptosis in vivo, we have produced a human-mouse (hu/mo) chimeric DR5 transgenic mouse. This mouse transgene is driven by the 3 kB mouse DR5 promoter and is regulated by a Floxed-STOP between the promoter and the hu/mo chimeric DR5 transgene. Thus, expression of hu/mo DR5 chimeric transgene can be targeted to synovial fibroblasts, B cells, T cells, or macrophages. In collaboration with Dr. Tong Zhou, we are analyzing the ability of a novel anti-human DR5 antibody (TRA8) to regulate arthritis and immune responses in these chimeric DR5 transgenic mice.
My laboratory has longstanding interest in age-related immune senescence. We were one of the first investigators to propose that T cell senescence is due to decreased, rather than increased, apoptosis. This was directly demonstrated using a CD2-Fas Tg mouse that resulted in increased expression of Fas throughout the lifespan of the mouse. This resulted in decreased T cell senescence. Our recent interest in T cell senescence is being carried out in a study of nonagenarians in collaboration with Dr. Michal Jazwinski (Tulane University) and Dr. Donald Scott (University of Pittsburgh). Nonagenarians are protected from immune senescence by several factors including increased levels of certain hormones, such as leptin and Insulin like growth factor binding protein 3(IGFBP3). Our ongoing studies are further characterizing methods to prevent immunosenescence with aging. This is relevant to preservation of immune responses tat may help prevent development of cancer, and provide adequate protection against viruses.
Moon Nahm, MD Pneumococcus is a major human pathogen causing pneumonia and is responsible for a large number of deaths worldwide among young children and old adults. Its virulence is mostly due to its carbohydrate capsule, which encases the pneumococci and shields them from the host immune system. Antibodies to the capsule can kill pneumococci and protect us from infections. Because our body can make these antibodies if the capsule is injected into us, the capsule is useful as a vaccine against pneumococcal infections. Pneumococci can express almost 100 types of capsule, but actual vaccines contain 10-23 common capsule types (serotypes). Following the widespread use of these vaccines however, serotypes absent from the vaccine have become more common, and vaccines have become less effective overall. To understand how pneumococci can evade vaccines, and to improve these vaccines, my laboratory studies diversity and immunity to the pneumococcus capsule.
Our studies of capsule diversity have led us to discover many new and important capsule types. Our discovery of serotype 6C showed how pneumococci can evade pneumococcal vaccines and has provided knowledge essential to vaccine design. Our discovery of 11E showed that innate immunity can recognize capsule and induce changes in capsule-making genes as pneumococci adapt to live in different body niches. It also showed that innate immunity can recognize variable pathogen structures and how pneumococci adapt to different niches of the body. We are now investigating how completely the capsule encases a bacterium. If the capsule leaves some areas of the pneumococcal surface exposed, then the molecules in the exposed areas can be useful as new vaccines. To do this, we are currently studying bacterial capsules and their synthesis with new super-resolution microscopy, which can visualize down to molecular dimensions. Our studies of immunity to the capsule showed that some vaccines can elicit non-functional antibodies. Thus, vaccine development can be simplified if antibodies can be tested directly for their function. We have developed a practical way to measure antibody function by inventing MOPA (Multiplexed OpsonoPhagocytic Assay). MOPA has become essential in improving pneumococcal vaccines and has simplified vaccine manufacture and evaluation. MOPA is now facilitating production of low cost pneumococcal vaccines available for the entire world. Our laboratory has been serving as a reference laboratory to the World Health Organization in this field.
Steffanie Sabbaj, PhD I am interested in studying T cells and their role in the protection, pathogenesis and transmission of various human pathogens, such as HIV-1, HCMV, Chlamydia and recently SARS-CoV-2. My focus is on different mucosal compartments with special emphasis on the female genital tract and breast milk. Our understanding of local mucosal immunity should aid in the development of preventative vaccines at mucosal surfaces. In addition, HIV-infection as a dysfunctional immunological state, can be used as a model to study human T cell immunology.
Harry W. Schroeder, Jr., MD, PhD Ultimately, it is the identity and specificity of the lymphocyte antigen receptor that determines the nature of the immune response to antigen. The mechanisms that underlie the diversification of the B- and T-cell antigen receptor repertoires appear to generate receptor diversity at random. However, repeated examples of near to absolute identity of receptor sequences between individuals suggest the existence of genetically programmed constraints that may be designed to bias the immune system to produce preferred, and perhaps optimal, repertoires. The implication is that violation of these programs could lead to immune dysfunction, and thus to disease. To test this hypothesis, we are developing mouse models wherein we force expression of altered, polyclonal repertoires that violate normal constraints on antigen receptor sequence or structure. In the first of these mice, where we have forced expression of arginine, histidine and asparagine in the HCDR3 interval of immunoglobulin H chains, we observed somatic selection against antigen binding sites that contained an excess number of these charged amino acids, yet the system ultimately failed to recapture the tyrosine and glycine residues normally encoded by wild-type germline sequence. B-cell development was impeded, immunity to influenza virus was impaired, and expression of IgG anti-DNA antibodies was enhanced. These results support the view that optimal distinction between self and non-self is a product of evolutionary selection.
Lisa M Schwiebert, PhD The major research interests of the laboratory include studying the physiology and pathophysiology of immune responses within the lung. These interests encompass the study of respiratory disorders in order to understand the cellular and molecular mechanisms that underlie airway inflammation. On-going projects examine how surface molecules, such as CFTR and CD40, regulate the airway epithelial expression of pro-inflammatory mediators, including chemokines and adhesion molecules, that initiate and exacerbate leukocyte migration. In addition, we are examining the anti-inflammatory effects of aerobic exercise on asthma-related immune responses. Through increased understanding of the mechanisms that trigger airway inflammation, we hope to develop novel therapeutic agents that combat airway inflammatory diseases such as cystic fibrosis and asthma.
Lewis Zhichang Shi, MD, PhD Identifying novel targets to improve immune checkpoint blockers. Our recent studies (Cell and Nature Communications, 2016) and previous reports demonstrated that immune checkpoint blockers (ICBs) (e.g., anti-CTLA-4) exert similar functional outcomes to those of common metabolic pathways (e.g., mTOR) (JEM and Immunity, 2011), i.e., promoting effector T cell (Teff) function (IFN-γ production) and depleting regulatory T cells (Treg). Interestingly, these effects are selectively induced in the tumor microenvironment , a special metabolic milieu with numerous features impacting the mTOR pathway. Given this key information, whether ICBs engage the mTOR pathways and its downstream targets in tumor- infiltrating T cell (TILs) is largely unknown and targeting these metabolic checkpoints as novel approaches to enhance therapeutic efficacy of ICBs remains to be explored. We will combine genetic mouse models with specific deletion of those genes in T cells, genetic manipulations with retroviral overexpression and CRISPR-CAS9 deletion, pharmacological approaches with metabolic inhibitors and activators, transplantable and orthotopic tumor models, and adoptive T-cell therapy (another promising modality in cancer immunotherapy) to evaluate therapeutic value of targeting these metabolic targets as a standalone therapy, or in combination with ICBs and conventional radiotherapy and chemotherapy. Further, we have an ongoing study showing that co- stimulatory molecule ICOS has an indispensable role in maintaining the survival and functionality of adoptively transferred tumor antigen-specific CD8+ T cells, especially when combined with ICBs. Currently, agonistic anti-ICOS therapeutic antibodies are being tested in Phase I clinical trials. Further mechanistic understanding will help guide these clinical trials and offer rationales to test additional combination therapies.
Functional and transcriptional control of T cell development and differentiation. The mTOR pathway is a master regulator for T cell metabolism, differentiation and function, with prominent roles in cancer, autoimmunity, and vaccination for infectious diseases. While the downstream targets of mTOR have been extensively studied, its upstream regulators are under-studied and an answer to which may offer potential therapeutic targets for various pathologies. Interestingly, TCR signaling regulates both Gfi1 expression and mTOR activity, suggesting Gfi1 and mTOR might crosstalk with each other. We will examine whether Gfi1 serves as an upstream regulator of the mTOR signaling. How the Gfi1-mTOR interaction dictates T cell development and acquisition of effector functions will be assessed using genetic mouse models and mouse autoimmune disease and tumor models. We recently showed that Gfi1 is required for anti-tumor immunity (PNAS, 2013) and for T cell maturation (PNAS, 2017). This study will offer new insights into whether mTOR pathway is the downstream link.