Faculty active in this area of research are listed below. For a brief description of their research interests, click on their name in the list. Clicking on the name at the beginning of the brief description links to their detailed personal website.
Susan L. Bellis, PhD The Bellis laboratory has two principal areas of research interest:
Role of receptor glycosylation in conferring a stem-like, apoptosis-resistant cell phenotype
Our laboratory has determined that the ST6Gal-I glycosyltransferase adds a sialic acid to a distinct subset of receptors, thereby imparting an undifferentiated cell phenotype. Using cell model systems and genetically engineered mice, we have shown that ST6Gal-I-mediated sialylation controls the function of select integrins, growth factor receptors and death receptors. Collectively these molecular pathways direct intracellular signaling cascades that regulate the migration and survival of both immune cells and epithelial tumor cells. In the case of tumor cells, upregulation of ST6Gal-I confers a cancer stem cell phenotype, and accelerates metastatic progression in animal models. The broad goal of our research is to elucidate the mechanistic basis for sialylation-dependent receptor signaling, and to determine whether manipulating sialylation levels can be used as a clinical treatment for pathologies such as autoimmune disorders and metastatic cancer.
Biomimetic scaffolds for bone repair
The goal of this project is to create bone-like synthetic matrices for bone regeneration using technologies such as electrospinning and 3D printing. The composite scaffolds produced by our group support robust mesenchymal stem cell survival and proliferation, and also stimulate substantial new bone formation when implanted into bone defects. To further enhance the osteoinductive properties of the substrates, the matrices are being functionalized with tissue regenerative factors such as PDGF and BMP-2. In complementary studies, we are developing novel methods for improving the bonding of bioactive proteins and peptides to bone-mimetic scaffolds. Our broad objective is to synthesize regenerative scaffolds that, when implanted, stimulate recruitment and osteoblastic differentiation of the patient’s mesenchymal stem cells, leading to accelerated bone regeneration.
Elizabeth E. Brown, PhD Using models of autoimmunity and immune-suppression, the work in our laboratory is targeted toward understanding the natural history of viral infections and aberrant immune function common to inflammatory-mediated chronic diseases. Of particular interest is the genetic basis of select host-pathogen interactions, virally-associated cancers, select lymphomas, systemic lupus erythematosus (SLE) and systemic vasculitis, each with underlying B cell pathologies. Within this purview, we use a multi-disciplinary functional genomics approach to explore pathways involved in chronic immune perturbation, B cell homeostasis, cytokine signaling as modifiers of disease, mucosal immunity and immune senescence as markers of complex disease susceptibility, morbidity and mortality. The goal of this research is to identify and validate molecular biomarkers of clinical outcomes, which may be used to target high-risk populations to prevent or reduce disease burden.
Randy Q. Cron, MD, PhD Host transcription factors exploited by HIV-1. HIV-1, the cause of AIDS, has infected over 40 million individuals world-wide. Although vast improvements in therapy have been developed over the last decade, HIV-1 cannot be totally eliminated from the host due to its ability to enter a resting or latent state in 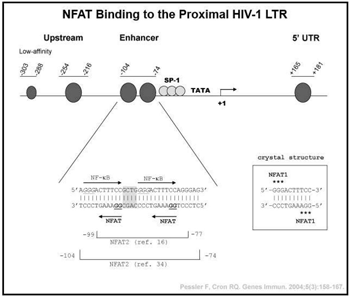 CD4 T cells. Because HIV-1 relies on host transcription factors to replicate, we are exploring the role of the calcium activated nuclear factor of activated T cells (NFAT) transcription factors in regulating HIV-1 transcription. We and others have shown that the CsA-sensitive NFAT proteins bind to the proximal HIV-1 promoter/long terminal repeat (LTR) in vitro and up-regulate HIV-1 transcription. We have further demonstrated that NFAT proteins bind to the integrated HIV-1 LTR in primary human CD4 T cells in vivo by chromatin immunoprecipitation, and this binding is disrupted by the regulatory T cell transcription factor, FOXP3. In addition, we are attempting to exploit NFAT activation as a means of activating HIV-1 LTR activity in latently infected cells. Recently, we identified a novel binding site for the c-maf transcription factor located adjacent to the proximal NFAT sites in the HIV-1 LTR. Our studies reveal synergistic transcriptional activation and increased infection of HIV-1 by c-maf, NFAT2, and NFΚB p65 in primary human IL-4-producing CD4 T cells. Thus, c-maf will likely be a novel therapeutic target in the treatment of HIV-1.
CD4 T cells. Because HIV-1 relies on host transcription factors to replicate, we are exploring the role of the calcium activated nuclear factor of activated T cells (NFAT) transcription factors in regulating HIV-1 transcription. We and others have shown that the CsA-sensitive NFAT proteins bind to the proximal HIV-1 promoter/long terminal repeat (LTR) in vitro and up-regulate HIV-1 transcription. We have further demonstrated that NFAT proteins bind to the integrated HIV-1 LTR in primary human CD4 T cells in vivo by chromatin immunoprecipitation, and this binding is disrupted by the regulatory T cell transcription factor, FOXP3. In addition, we are attempting to exploit NFAT activation as a means of activating HIV-1 LTR activity in latently infected cells. Recently, we identified a novel binding site for the c-maf transcription factor located adjacent to the proximal NFAT sites in the HIV-1 LTR. Our studies reveal synergistic transcriptional activation and increased infection of HIV-1 by c-maf, NFAT2, and NFΚB p65 in primary human IL-4-producing CD4 T cells. Thus, c-maf will likely be a novel therapeutic target in the treatment of HIV-1.
Genetic defects in lymphocyte cytolysis in macrophage activation syndrome. Macrophage activation syndrome (MAS) is a hyper-inflammatory immune response in children and adults that is often triggered by certain infectious (e.g. EBV), autoimmune (e.g. lupus), autoinflammatory (e.g. Still disease), and oncologic (e.g. T cell leukemia) disorders. MAS results in pro-inflammatory cytokine storm leading to pancytopenia, coagulopathy, central nervous system dysfunction, and multi-organ system failure. MAS is frequently lethal like its cousin disease familial hemophagocytic lymphohistiocytosis (fHLH). fHLH is uniformly fatal if not treated aggressively and typically presents in the first few months of life in infants 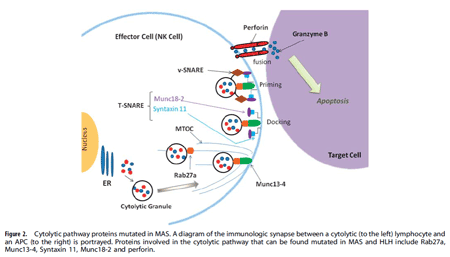 with bi-allelic genetic defects in one of the proteins involved in perforin mediated cytolysis by natural killer (NK) cells and CD8 cytotoxic lymphocytes. Recently, mono-allelic (heterozygous) mutations in cytolytic pathway proteins (e.g. perforin, Munc13-4, Rab27a, etc.) have been identified in a substantial percentage of MAS patients presenting beyond infancy. In our MAS patient cohort, we have identified several mutations, including novel mutants, in a variety of cytolytic pathway genes. Using lentiviral transduction of mutant and wild-type genes into NK cells, we demonstrate decreased cytolytic activity by over-expression of the mutant genes, suggesting a partial dominant-negative effect. These studies suggest that there are likely genetic predispositions to develop MAS, and we are currently exploring the novel mutations and their pathophysiological consequences on lymphocyte mediated cytolytic function.
with bi-allelic genetic defects in one of the proteins involved in perforin mediated cytolysis by natural killer (NK) cells and CD8 cytotoxic lymphocytes. Recently, mono-allelic (heterozygous) mutations in cytolytic pathway proteins (e.g. perforin, Munc13-4, Rab27a, etc.) have been identified in a substantial percentage of MAS patients presenting beyond infancy. In our MAS patient cohort, we have identified several mutations, including novel mutants, in a variety of cytolytic pathway genes. Using lentiviral transduction of mutant and wild-type genes into NK cells, we demonstrate decreased cytolytic activity by over-expression of the mutant genes, suggesting a partial dominant-negative effect. These studies suggest that there are likely genetic predispositions to develop MAS, and we are currently exploring the novel mutations and their pathophysiological consequences on lymphocyte mediated cytolytic function.
Steven R. Duncan, MD Research interests of Dr. Duncan center on parsing out the immunological mechanisms involved in the pathogenesis of some morbid and mostly refractory chronic lung diseases, particularly idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease. Ongoing studies include those to better understand the processes by which human T-cells undergo genomic, phenotypic, and functional changes after repeated antigen encounters, and explorations of ways to specifically target these cells or interfere with their functions. A recently developed human-chimeric mouse model, in which these animals are reconstituted with a human adaptive immune system will be helpful. Other investigations in progress include more detailed characterization of the autoantibody repertoires in these disease populations, with the aim of identifying autoantibody (and T-cell) specificities with greatest utility in diagnostic or prognostic assays. Additional, interrelated projects include further explorations of recently discovered mechanisms by which T-cells regulate fibroblast production of extracellular matrix, and high resolution sequencing (and functional studies) of novel immunogenetic regulatory polymorphisms that confer high risks of developing these chronic lung diseases. In addition to these bench studies, we plan to continue and extend early phase trials of novel immunological response modifiers in these patients.
Charles O. Elson III, MD The central focus of the laboratory is on the regulation of mucosal immune responses, particularly the mucosal immune response to the microbiota, which represent the largest mass of antigen encountered by the immune system. The cellular and molecular mechanisms that maintain mucosal immune homeostasis are being defined. When these mechanisms fail, pathogenic effector T cells are generated that result in colitis. We have cloned a set of immunodominant antigens of the microbiota that stimulate such pathogenic T cells and result in inflammatory bowel disease. Among these cloned antigens, previously unknown bacterial flagellins have emerged as a major cluster. Seroreactivity to these flagellins is found in multiple experimental models of colitis in mice and in half of patients with Crohn's disease. These antigens drive a newly described CD4 T cell effector subset making IL-17 (Th17) which appears to be responsible for disease progression. A T cell receptor transgenic mouse reactive to CBir1 flagellin has been generated and is being used to study the innate and adaptive immune response to these microbiota antigens. A second major effort is in the identification of T reg cells in the intestine that recognize microbial antigens and maintain homeostasis. The mechanisms whereby such cells are induced are being defined and the application of these cells to prevent or treat intestinal inflammation is being tested. Lastly, a microbiota antigen microarray has been constructed which can be used to analyze serologic reactivity to the microbiota in both mouse and human. Sera from various human populations are presently being analyzed.
Hui-Chen Hsu, PhD Two major studies are currently ongoing in my laboratory:
1. We have identified that autoimmune BXD2 mice exhibit unique features, including spontaneous formation of germinal centers, increased expression of activation-induced cytidine deaminase (AID), increased production of pathogenic autoantibodies that are polyreactive, significantly increased percentage of IL-17high CD4 TH cells (TH-17) and IL-17Rhigh B cells, and significantly increased numbers of type I interferon producing plasmacytoid dendritic cells in the spleens of these mice. We are currently studying the interconnection of high IL-17, high type I IFN and the development of spontaneous germinal centers in these mice.
2. We are developing a new lupus mouse model to study the safety and efficacy of using an anti-human DR5 antibody (TRA-8) as novel therapy of lupus and other autoimmune diseases. Death receptor 5 (DR5) is a cell surface receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Investigators at UAB (Dr. Tong Zhou and colleagues) have generated a unique anti-human DR5 antibody (TRA-8) that triggers the death of DR5+ cells. TRA-8 was selected due to its signaling of apoptosis (differing from TRAIL, which can induce proliferation). We have found that it kills cultured human lupus CD4+ T cells and plasma B cells. We have developed a transgenic mouse model that expresses a Floxed-STOP humanized DR5 mouse transgene (hu/mo DR5 Tg) and will express this hu/mo DR5 Tg in T and B cells in autoimmune mice that develop lupus-like disease. The ongoing project is to test the method of action and effectiveness of TRA-8 in depleting autoreactive CD4 T and plasma B cells, and its safety, in a special humanized mouse model, to determine its potential utility as a therapy for patients with lupus.
Hui Hu, PhD Utilizing a broad variety of techniques including cellular immunology, molecular biology, biochemistry, gene-targeting (knockout and knockin), functional genomics and in vivo animal models, the Hu laboratory is interested in identifying novel regulatory genes and transcriptional networks that play critical roles in regulating the adaptive immunity. One of the research projects in the Hu laboratory is to study T follicular helper (Tfh) cells and germinal center (GC) responses (Nat. Immunol. 2014). The complex regulation that determines the initial development of Tfh cells, their developmental progression in germinal centers, and their fates after an immune response dissolves, is still not fully understood. The Hu laboratory is interested in identifying novel pathways underlying the differentiation of Tfh cells in humoral responses and designing new strategies to manipulate humoral responses for treatment of infectious diseases and autoimmune disorders. The Hu laboratory is also working to find ways to activate T cells under immunosuppressive circumstances. The Hu laboratory has demonstrated that cell-intrinsic signaling pathways are required to maintain mature T cells in a quiescent state. If these pathways are disrupted, resting T cells become aberrantly activated even in the absence of antigen challenge (Nat. Immunol. 2011). The Hu laboratory is interested in identifying regulatory genes and pathways that actively restrain T cell activation, and defining the roles of such negative regulatory pathways in controlling T cell quiescence, effector responses, memory maintenance, and tumor immunology.
Janusz H. Kabarowski, Ph.D. Dr. Kabarowski’s research program is focused on the study of lipids and lipoprotein metabolism in chronic inflammatory disease (notably atherosclerosis and autoimmune disease). Early work characterized the role of the G2A lipid receptor in atherosclerosis and lipoprotein metabolism, showing that pro-atherogenic effects of this receptor may be mediated through its modulatory influence on hepatic High-Density Lipoprotein (HDL) biogenesis. More recently, Dr. Kabarowski’s group described autoimmune-mediated effects on HDL metabolism in normolipidemic mouse models of Systemic Lupus Erythematosus (SLE) and currently a major effort of his laboratory is directed toward developing therapeutic approaches by which anti-inflammatory and immunosuppressive properties of HDL may be harnessed to improve major Lupus phenotypes and combat premature atherosclerosis, a major cause of morbidity and mortality in this and other rheumatic autoimmune diseases. Emphasis is placed on determining the mechanisms by which protective anti-inflammatory properties of HDL are subverted by chronic inflammation, understanding how this influences immunoregulatory processes involved in SLE and atherosclerosis, and establishing the therapeutic efficacy of HDL-targeted approaches such as HDL mimetic peptides in SLE and other autoimmune diseases.
Robert P. Kimberly, MD Our laboratory is interested in the role of genetic factors in the normal function of the immune system and in development of autoimmune and immune-mediated inflammatory diseases such as systemic lupus erythematosus and systemic vasculitis. Our approach has focused on receptors for immunoglobulin (Fc receptors) as a model system and has explored molecular mechanisms of receptor signaling and the molecular basis for receptor polymorphisms in humans. Studies in cell lines and in normal donors have demonstrated that despite the common theme of receptor-induced tyrosine phosphorylation, the various human Fc receptors engage different signaling elements which are reflected in important distinctions in function. Similarly, allelic variations in receptor structure profoundly affect receptor function, and certain low-binding alleles are enriched in SLE patients. More active alleles are over-represented in patients with vasculitis and severe renal disease. Other genes and gene families are being pursued as they are identified as candidate genes through genome wide association studies. These genes include complement receptors, cytokine genes and their promoters, signal transduction molecules, and members of the TNF superfamily.
Frances E. Lund, PhD The overarching research objective of the Lund laboratory is to identify the key players that suppress or exacerbate mucosal inflammatory responses with the long-term goal of developing therapeutics to treat immunopathology associated with chronic infectious, allergic and autoimmune disease. One of the lab’s major projects is to characterize the roles that cytokine-producing “effector” B cells play in modulating inflammation and T cell-mediated immune responses to pathogens, autoantigens and allergens. In a second project, the lab evaluates how inflammatory signals regulate the balance between the development of the antibody-producing long-lived plasma cells and the memory B cell compartment within lymphoid tissues. The lab also studies how these cells are maintained long-term at inflammatory sites. Finally, the lab examines how oxidative stress induced by reactive oxygen species impacts inflammation, immune responses and cellular metabolism. In particular, the lab is experimentally modulating the NAD metabolome of immune cell in order to alter the responsiveness of these cells to oxidative stress.
John D. Mountz, MD, PhD A hallmark of autoimmune disease is the development of autoantibodies that can cause disease. My laboratory has identified that the second recombinant inbred strain of B6 x DBA/2 (BXD2) spontaneously produces very high levels of pathogenic autoantibodies. Single antibodies produced by hybridomas from spleens of these mice transfer arthritis or glomerulonephritis in normal mice. By 3 months of age, the spleens of BXD2 mice are greatly enlarged and are packed with numerous large, spontaneous germinal centers (GCs). This GC development is promoted by high levels of Th17 and IL-17 in these mice. IL-17 signals through the IL-17a receptor in B cells resulting in increased classical NF-κB pathway activation. This activates several genes, including regulators of G-protein signaling (RGS) 13 and 16. Upregulation of RGS genes impairs signaling through CXCR4/CXCL12 and CXCR5/CXCL13 to arrest migration and movement of T cells and B cells. This enables prolonged and stable interaction of B cells and CD4 T cells. Key ongoing questions in my laboratory include what is the mechanism for increased Th17 development. IL-6 is highly produced by B cells, macrophages and plasmacytoid dendritic cells (PDCs). TGF-β, however, is not greatly increased. What are the factors, in combination with IL-6, that promote high Th17 development in BXD2 mice? How does Th17 signal through B cells? Our recent evidence indicates that IL-17 signaling requires both TRAF6 and ACT1, which has been identified in IL-17 signaling pathways. Current ongoing work is to determine the mechanism of increased NF-κB signaling in response to IL-17 in B cells. Also using RGS13 KO and RGS16 KO mice, we wish to determine which of these RGS proteins is highly essential for development of spontaneous autoreactive GCs. We also wish to identify the most promising points for interruption of IL-17 signaling that upregulates RGS expression in B cells. Other studies include detailed analysis of the effect of IL-17 on B cell chemotaxis in response to CXCL12 and CXCL13. These include in vitro chemotactic chamber analysis, and live imaging analysis using confocal microscopy.
A second area of interest is the role of DR5 apoptosis in arthritis and autoimmune Disease. TRAIL-DR5 apoptosis signaling is very similar to FAS apoptosis signaling involving mitochondrial amplification loop and Bcl-2 family members, as well as direct induction of apoptosis through caspase activation resulting in terminal caspases 3, 5, and 7 activation. The TRAIL-DR5 apoptosis signaling pathway, like Fas, is inhibited by FLIP-L and XIAP (inhibitors of apoptosis proteins). DR5 is upregulated on synovial fibroblasts of patients with rheumatoid arthritis and in Collagen-II mouse model of arthritis. To determine mechanisms of DR5 apoptosis in vivo, we have produced a human-mouse (hu/mo) chimeric DR5 transgenic mouse. This mouse transgene is driven by the 3 kB mouse DR5 promoter and is regulated by a Floxed-STOP between the promoter and the hu/mo chimeric DR5 transgene. Thus, expression of hu/mo DR5 chimeric transgene can be targeted to synovial fibroblasts, B cells, T cells, or macrophages. In collaboration with Dr. Tong Zhou, we are analyzing the ability of a novel anti-human DR5 antibody (TRA8) to regulate arthritis and immune responses in these chimeric DR5 transgenic mice.
My laboratory has longstanding interest in age-related immune senescence. We were one of the first investigators to propose that T cell senescence is due to decreased, rather than increased, apoptosis. This was directly demonstrated using a CD2-Fas Tg mouse that resulted in increased expression of Fas throughout the lifespan of the mouse. This resulted in decreased T cell senescence. Our recent interest in T cell senescence is being carried out in a study of nonagenarians in collaboration with Dr. Michal Jazwinski (Tulane University) and Dr. Donald Scott (University of Pittsburgh). Nonagenarians are protected from immune senescence by several factors including increased levels of certain hormones, such as leptin and Insulin like growth factor binding protein 3 (IGFBP3). Our ongoing studies are further characterizing methods to prevent immunosenescence with aging. This is relevant to preservation of immune responses tat may help prevent development of cancer, and provide adequate protection against viruses.
Jan Novak, PhD Research interests involve a wide area of biologically active compounds of natural origin (multidisciplinary approaches to identification, isolation, and analyses of novel compounds, studies on their structure, biosynthesis, and genetics, mode of action and mechanism of resistance), biochemistry and genetics of post-translationally modified peptides and proteins, enzymes and pathways of primary and secondary metabolism and cellular regulations, intercellular communication and signaling, and glycosylated compounds. Of particular interest more recently are studies on glycosylation of immunoglobulins in health and disease in humans (IgA nephropathy, chronic inflammatory diseases, Kawasaki syndrome) and regulation of immunoglobulin glycosylation.
The hallmark of IgA nephropathy (IgAN), the most common glomerulonephritis in the world, is deposition of IgA1-containing immune complexes into the glomerular mesangium. Proliferation of mesangial cells (MC) and extracellular matrix (ECM) expansion occurs from early stages, progressing into glomerulosclerosis and development of end stage renal disease. High levels of IgA1-containing circulating immune complexes (CIC) are often observed in IgAN patients indicating a defect in CIC clearance. Galactose (Gal) -deficient O-glycans were detected in the hinge region of IgA1 molecules in CIC in IgAN patients. These Gal-deficient IgA1 molecules are complexed with IgG (IgA1) antibodies with anti-GalNAc specificity. Importantly, Gal-deficient IgA1 is also found in kidney immune deposits in IgAN patients. Dr. Novak's group hypothesizes that the glycosylation aberrance of a fraction of IgA1 molecules results in formation of CIC that ultimately deposit in the mesangium, leading to IgAN. Based on preliminary results, they postulate that the CIC bind to MC through a novel IgA receptor and possibly other receptors, and trigger signaling events resulting in proliferation of MC and ECM expansion. The group has studied interactions of CIC with MC using various approaches, including for example confocal laser scanning microscopy, differential gene and protein expression using DNA arrays and proteomics approaches, respectively. The ultimate goal of these studies is to understand how CIC form, what are major factors inducing aberrant IgA glycosylation, and how CIC trigger pathological response of MC leading to IgAN. We are hopeful that a better understanding of this chronic disease may open new ways for diagnosis or even treatment.
Chander Raman, PhD Dr. Raman’s research interrogates molecular and cellular mechanisms driving the immunopathogenesis of autoimmune diseases with a special emphasis on multiple sclerosis (MS) and rheumatoid arthritis (RA). Within this context, the research interest of the Raman laboratory is the study of activation and differentiation of effector T cells and B cells in the pathogenesis of these autoimmune disease. Current investigations involve human samples from patients with MS or RA as well mouse models to study these diseases. The major areas of investigation are:
- The mechanisms modulating the activation of T-cells and differentiation to pathogenic (Th1, Th17 and ThIFNγIL-17 –dual producers), regulatory (nTreg, iTreg) Th subsets and cells of the innate immune system (dendritic cells, macrophages and microglia). Within this area of study, the Raman laboratory has a special interest in type 1 and type 2 interferons, and TGFβ family proteins in the pathogenesis of MS, RA and the mouse model, experimental autoimmune encephalomyelitis (EAE)
- Molecular mechanisms by which CK2 and GSK3 modulates effector and regulatory cells in the pathogenesis of autoimmunity
- Role of CD5 in T cell and B-1a B cell development, differentiation, immunity and pathogenesis – the laboratory focuses on B-1a B cell-dependent T-independent antibody responses, T-dependent antibody responses, autoreactive B-cell generation and persistence and regulatory B-cells. For these studies, the Raman laboratory has generated unique knock-in CD5 mutant mice in which signaling domains associated with CD5-inhibitory activity (ITIM) and CD5-CK2 activation have been ablated
- TGFβR3/betaglycan dependent regulation of adaptive immune effector cells in the pathogenesis of autoimmune diseases
Lewis Zhichang Shi, MD, PhD Identifying novel targets to improve immune checkpoint blockers. Our recent studies (Cell and Nature Communications, 2016) and previous reports demonstrated that immune checkpoint blockers (ICBs) (e.g., anti-CTLA-4) exert similar functional outcomes to those of common metabolic pathways (e.g., mTOR) (JEM and Immunity, 2011), i.e., promoting effector T cell (Teff) function (IFN-γ production) and depleting regulatory T cells (Treg). Interestingly, these effects are selectively induced in the tumor microenvironment , a special metabolic milieu with numerous features impacting the mTOR pathway. Given this key information, whether ICBs engage the mTOR pathways and its downstream targets in tumor-infiltrating T cell (TILs) is largely unknown and targeting these metabolic checkpoints as novel approaches to enhance therapeutic efficacy of ICBs remains to be explored. We will combine genetic mouse models with specific deletion of those genes in T cells, genetic manipulations with retroviral overexpression and CRISPR-CAS9 deletion, pharmacological approaches with metabolic inhibitors and activators, transplantable and orthotopic tumor models, and adoptive T-cell therapy (another promising modality in cancer immunotherapy) to evaluate therapeutic value of targeting these metabolic targets as a standalone therapy, or in combination with ICBs and conventional radiotherapy and chemotherapy. Further, we have an ongoing study showing that co-stimulatory molecule ICOS has an indispensable role in maintaining the survival and functionality of adoptively transferred tumor antigen-specific CD8+ T cells, especially when combined with ICBs. Currently, agonistic anti-ICOS therapeutic antibodies are being tested in Phase I clinical trials. Further mechanistic understanding will help guide these clinical trials and offer rationales to test additional combination therapies.
Functional and transcriptional control of T cell development and differentiation. The mTOR pathway is a master regulator for T cell metabolism, differentiation and function, with prominent roles in cancer, autoimmunity, and vaccination for infectious diseases. While the downstream targets of mTOR have been extensively studied, its upstream regulators are under-studied and an answer to which may offer potential therapeutic targets for various pathologies. Interestingly, TCR signaling regulates both Gfi1 expression and mTOR activity, suggesting Gfi1 and mTOR might crosstalk with each other. We will examine whether Gfi1 serves as an upstream regulator of the mTOR signaling. How the Gfi1-mTOR interaction dictates T cell development and acquisition of effector functions will be assessed using genetic mouse models and mouse autoimmune disease and tumor models. We recently showed that Gfi1 is required for anti-tumor immunity (PNAS, 2013) and for T cell maturation (PNAS, 2017). This study will offer new insights into whether mTOR pathway is the downstream link.
Matthew Stoll, MD, PhD, MSCS My major research interest is the link between mucosal immunity and spondyloarthritis. Specifically, I am looking at the adaptive (humoral and T cell) immune responses to enteric organisms and the nature of the fecal flora in patients with spondyloarthritis. I am also interested in the epidemiology, diagnosis, and treatment of temporomandibular joint (TMJ) arthritis in children with juvenile idiopathic arthritis (JIA).
Jianming (James) Tang, DVM, PhD Dr. Tang's ongoing research focuses on genetic and epigenetic contributions to infection and immunity in human populations. Immunogenetic studies deal with genes that govern innate and adaptive immune responses to infectious diseases or vaccination. Epigenetic analyses target CpG methylation and microRNA. Disease models range from HIV/AIDS to malignant glioma.
Hubert Tse, PhD The overall research objective in the Tse laboratory is to define and prevent immune-mediated effector mechanisms involved in the destruction of insulin-producing pancreatic beta-cells in autoimmune Type 1 diabetes (T1D). An overarching theme in our research is to determine the involvement of oxidative stress and the generation of reactive oxygen species (ROS) as effector and signaling molecules in autoimmune and pro-inflammatory-mediated diseases (Collagen-Induced Arthritis (CIA), Experimental Autoimmune Encephalomyelitis (EAE), Spinal Cord Injury, Traumatic Brain Injury). Research from our lab and others has shown that efficient T cell activation requires three signals mediated by antigen-presenting cell and naïve T cell interactions: signal 1 (T cell receptor – MHC), signal 2 (co-stimulatory molecules), and signal 3 (ROS and pro-inflammatory cytokines). To corroborate the importance of ROS-dependent signaling (signal 3) in T1D, a dominant negative p47phox (Ncf1m1J) mutation of the NADPH oxidase complex was introgressed into the non-obese diabetic (NOD) mouse, a murine model for studying Type 1 diabetes. NOD.Ncf1m1J mice are impaired in ROS synthesis and highly resistant to spontaneous diabetes and adoptive transfer of diabetes with diabetogenic T cells. CD4+ and CD8+ T cells are the final effector cells involved in pancreatic beta-cell destruction. Pro-inflammatory macrophages are equally important, as they constitute the first immune cells recruited into pancreatic islets to initiate beta-cell destruction and to activate naïve diabetogenic T cells. Currently, we seek to understand the synergy of oxidative stress and ROS synthesis on the activation of innate immune cells to diabetogenic viral triggers (Coxsackie B4, Encephalomyocarditis virus) and autoreactive T cells in murine models and human translational studies of Type 1 diabetes.