 Professor of Microbiology
Professor of Microbiology
| Address: | 1825 University Blvd Shelby Building, room 513 UAB Birmingham, AL 35294-2182 |
| Telephone: | (205) 934-6529 |
| FAX: | (205) 996-9908 |
| Email: | peterb@uab.edu |
| Publications |
________________________________________________________
Education
B.S., University of Massachusetts, Amherst
Ph.D., University of Alabama at Birmingham
Post-doctoral Fellow, Friedrich-Miescher Laboratory of the Max-Planck Institute, Tubingen, Germany
Research Interests
The immune system provides unique opportunities for the study of human diseases and for the understanding of basic processes in developmental biology. Immune cells undergo characteristic phenotypic and morphologic changes during their life span, and they do so at different places in the body, thus migration and homing are essential features of this developmental pathway. The lymphocytes of the immune system are unique in that changes in genotype to generate antigen receptor genes are required for their normal development. Three basic cell types required for adaptive immunity are T and B lymphocytes and antigen presenting cells. To generate an effective immune response, these cells must interact with one another via cell surface receptors and soluble mediators. The resulting signal transduction events may have multiple outcomes including induction of proliferation, differentiation, or programmed cell death (apoptosis).The long term goal of our studies is to understand the normal progression of the multipotential hemopoietic stem cell as it commits to development along the B cell lineage, undergoing the changes in phenotype and genotype that are characteristic of B cells.
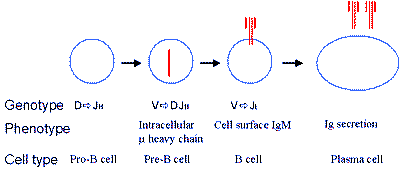 Figure 1. B cell development
Figure 1. B cell development
Uncommitted progenitor cells undergo sequential changes in the structure of their genomes, beginning first at the heavy chain locus (VDJH) to allow production of µ heavy chains. These associate with lambda5 and VpreB chains to form a pre-B cell receptor (not shown). Following successful light chain gene rearrangement (VJL), monomeric IgM is expressed on the surface of the B cell. Stimulation by cognate antigen with T cell help leads to the generation of antibody-secreting plasma cells.
Function of a novel intracellular Fc receptor related protein in B cells
We have identified FcRLA as a new member of the larger Fc receptor (FcR) and FcR-like (FcRL) gene family of humans and mice (Reference). Among hemopoietic cells, FcRLA expression is restricted to B cells. In humans, FcRLA is preferentially expressed by germinal center B cells but its expression in the mouse has not yet been defined. The conventional FcR and the FcRL molecules are nearly all transmembrane proteins, and all of them have extracellular glycoprotein domains for ligand binding. In striking contrast, FcRLA is an intracellular protein. It lacks N-linked glycosylation sites and a transmembrane region, but contains a signal sequence common to proteins targeted to the endoplasmic reticulum (ER). Our data now clearly indicate a role for FcRLA as a resident ER protein that associates with immunoglobulin in this organelle. Moreover, analysis of FcRLA expression in the Ramos IgM B cell line, which produces both the membrane (m) and secretory (s) isoforms of μ heavy chain, indicates that FcRLA preferentially associates with μs. The unique features and restricted cellular distribution of FcRLA suggest its importance in the metabolism of Ig in B cells and in normal immune system function. Definition of the composition and function of the Ig-FcRLA complex is a major goal of our studies. These functions could include aiding Ig folding, facilitating Ig assembly, regulating Ig transport, and/or monitoring the degradation of incompletely processed Ig molecules. FcRLA may play an essential role in the intracellular quality control system that ensures the correct production of antibodies. Moreover, its preferential expression in human germinal center B cells, where B cell proliferation, antibody class switching, and mutation of antibody variable region genes occurs, suggests that defects in FcRLA expression may lead to autoimmunity or immunodeficiency diseases.
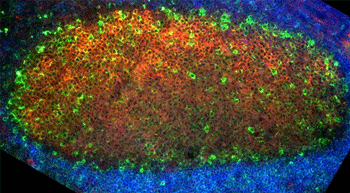
Figure 2. A section of human tonsil stained for FcRLA (green), PNA (orange), a marker for germinal center B cells, and IgD (blue) a marker for follicular B cells