-
Current on-boarding process for new studies
The first step in establishing a new study with CINL is to complete and submit our Inquiry and Quote Request forms.
-
New Study Inquiry Form
Please fill out this short form to provide basic information about a new study.
Please email
This email address is being protected from spambots. You need JavaScript enabled to view it. after you submit the forms. Feel free to reach out to us with questions. -
Quote Request
For pricing and specifics, please complete the Quote Request form located at the bottom of the UAB HISF Activity page “MRI Only (Pre-Clinical/Basic Science) Review/Quote Request.” This form launches a more in-depth review of a proposed study and therefore will take more time to complete and submit. It includes a request for a PDF of the protocol, however an idea of the sequences and total scan length should suffice temporarily until a complete protocol is in place. Initially, a PI can review the form and submit it after the required fields are completed.
-
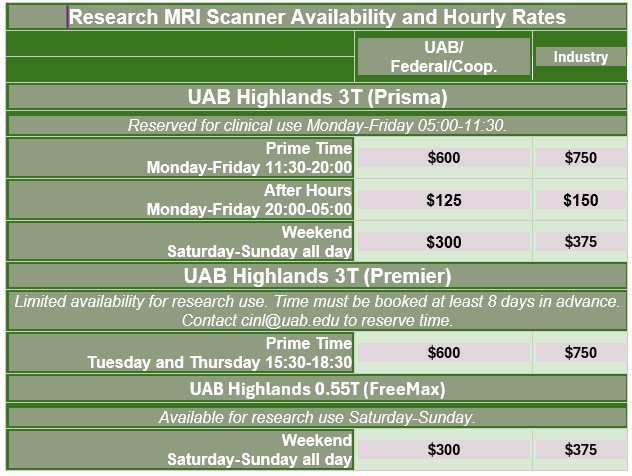
Pricing
-
Development Hours
Each study receives up to 5 hours of development time for protocol setup and testing purposes. Follow our Calendar instructions (linked) for requesting this time keeping in mind to indicate the P-number along with PI name(s) and “development” when scheduling development time on the scan calendar. Once your study begins, CINL will not cover the cost of continued development time. If you believe you will need more than 5 hours of development time, or if you will require continued development time over the course of your study, please consider applying for the Radiology/CCTS Imaging Development Voucher Program.