| 2009 Case #3 |  |
|
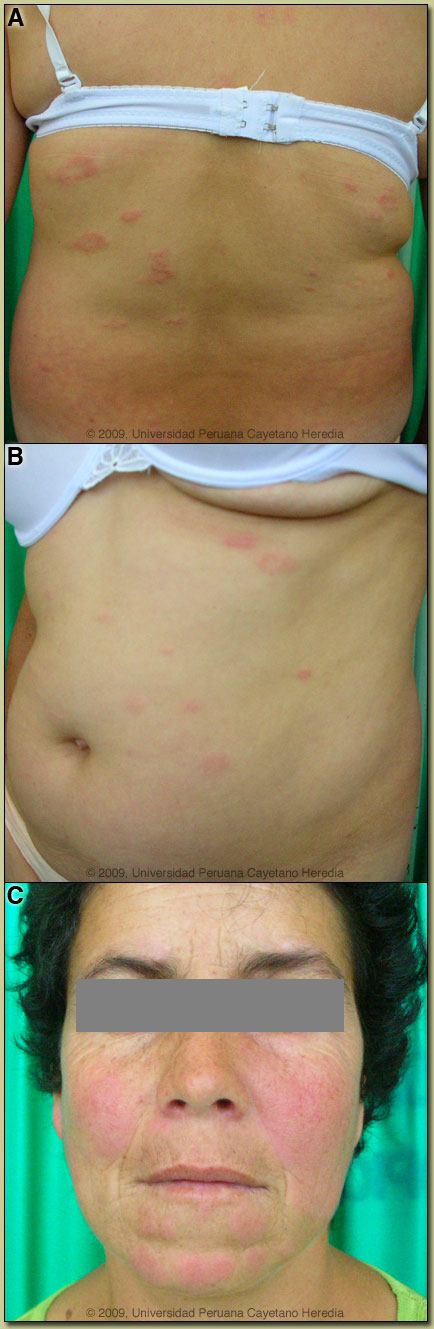
| Diagnosis: Mycobacteria leprae. Multibacillary leprosy according to the WHO classification. Borderline tuberculoid (BT) leprosy according to the Ridley-Jopling classification. |
 Discussion: The skin lesions with impairment of sensation in this patient make a clinical diagnosis of leprosy even without the biopsy or AFB results. In addition she has motor peripheral nerve involvement, which further substantiates this diagnosis. If there were no impairment of sensation other conditions to consider in the differential diagnosis could be an allergic reaction, but in this case the history is probably too long and there is no pruritus; secondary syphilis; sarcoidosis; disseminated cutaneous leishmanias; or cutaneous lymphoma. Discussion: The skin lesions with impairment of sensation in this patient make a clinical diagnosis of leprosy even without the biopsy or AFB results. In addition she has motor peripheral nerve involvement, which further substantiates this diagnosis. If there were no impairment of sensation other conditions to consider in the differential diagnosis could be an allergic reaction, but in this case the history is probably too long and there is no pruritus; secondary syphilis; sarcoidosis; disseminated cutaneous leishmanias; or cutaneous lymphoma.
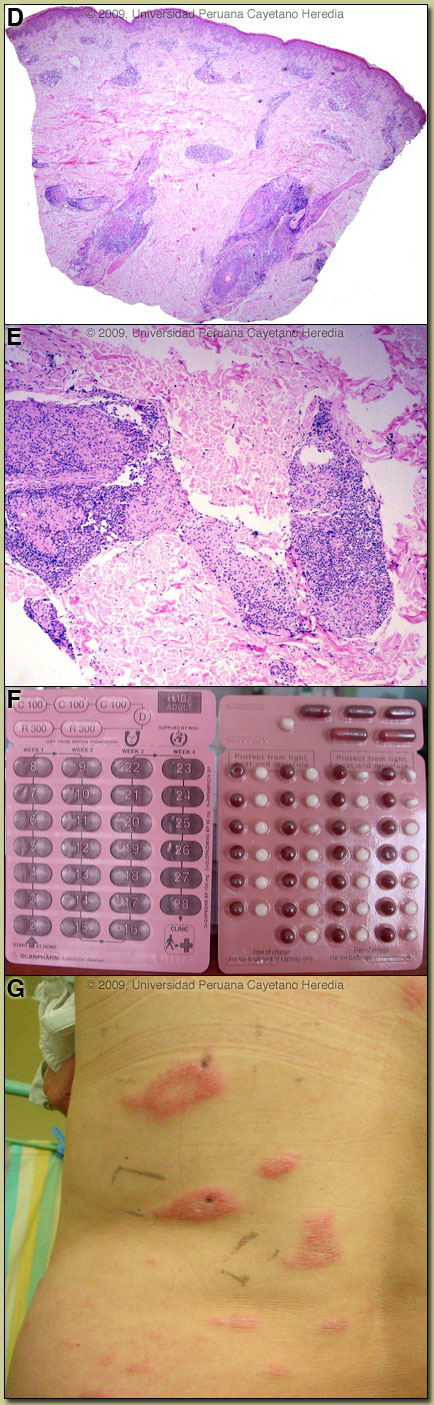
In this patient, we obtained slit skin smears stained for acid-fast bacilli with the following results: negative from both earlobes, elbows, knees and from 3 plaques. Slit skin smears are performed by making small (5mm length, 2mm depth) slits in pinched skin (to avoid bleeding), the edges of which are scraped. The material obtained is smeared on a clean slide and stained for AFB. Generally ear lobes, elbows, knees and one or two lesions are examined. The bacterial index ranges from zero (no bacillli in 100 oil-immersion fields) to 6+ (over 1000 bacilli in one field). A biopsy of a plaque showed a superficial and deep perivascular and periadnexal infíltrate with a tendency to a linear array [Image D]. At higher power the granulomatous nature of the infiltrate becomes clear with an elongated shape to the granulomas [Image E]. The centers of the tuberculoid granulomas are mainly populated by epithelioid histiocytes, whereas the periphery consists of mature lymphocytes. A granulomatous infiltrate, in a linear pattern, involving the reticular dermis is consistent with a diagnosis of leprosy, closer to the tuberculoid pole of the spectrum and representing Borderline Tuberculoid disease (classification discussed below). The usual and most practical grading system is the WHO classification. For therapeutic purposes, it matters only whether the patient has paucibacillary or multibacillary disease. Where no slit skins smears can be done, paucibacillary leprosy is defined as 5 or fewer skin lesions; multibacillary cases have 6 or more lesions. Paucibacillary disease usually presents with small numbers of hypopigmented erythematous macules or plaques with absent sensation, well-demarcated borders, and some scaliness. Multibacillary disease is usually widespread at diagnosis with non-anesthetic infiltrated areas of skin with indistinct borders, non-anesthetic papules or nodules. The disease can be classified precisely in the immunologic sense using the traditional Ridley-Jopling classification. This is a spectrum of disease ranging from tuberculoid leprosy (TT) with no or few AFB in lesions and good cell mediated immunity, to lepromatous leprosy (LL) with many AFB and poor cell-mediated immunity. Borderline tuberculoid (BT), borderline borderline (BB), and borderline lepromatous (BL) represent the unstable evolving stages of the disease. The erythematous plaques with wide areas of spared skin are compatible with borderline tuberculoid leprosy (BT), but there are too many lesions, (more than 100), which put her somewhere in the spectrum between BT and mid borderline leprosy (BB). The lesions with a tendency to punched-out lesions are also typical of mid borderline leprosy. Because leprosy in the middle of this spectrum is unstable, patients tend to move along the spectrum. In this case, because most of the lesions are plaques and only few show the punched-out feature, and because the histopathology shows characteristics of BT, our patient is probably moving towards the BB (mid borderline) side of the spectrum. Records were obtained indicating that the patient had been diagnosed with BT evolving to BB leprosy 3 years earlier with similar skin lesions (also more than 100) and hypoesthesia at the tips of all fingers and toes. Hypoesthesia was also present at that time on the ulnar aspect of the left forearm and lateral aspect of the left leg. Slit skin smears from the earlobes, elbows and knees were negative, but 1+ acid fast bacilli were found from one of the lesions. A skin biopsy was compatible with BT leprosy and it also had 1+ AFB. She was treated with a multibacillary regimen of dapsone + rifampin + clofazimine for one year due to the number of lesions and the presence of AFB though at low levels. Lesions disappeared and sensation was restored. Leprosy is a disease of peripheral nerves and skin [reviewed in Lancet. 2004 Apr 10;363(9416):1209-19 and Clin Dermatol. 2007 Mar-Apr;25(2):165-72]. Peripheral nerves such as the ulnar, median, common peroneal, posterior tibial, facial, and greater auricular are often palpably enlarged. The current patient did not have any evidence of thickened peripheral motor nerves on physical examination. In advanced neuropathy this often leads to motor deformities such as claw hand, footdrop, hammer or claw toes, and hand and foot insensitivity. The standard WHO regimen for paucibacillary disease is 100mg Dapsone a day unsupervised and 600mg Rifampin once per month directly observed for 6 months. For multibacillary disease patients receive 100mg Dapsone and 50mg Clofazimine a day unsupervised and 600mg Rifampin and 300mg of Clofazimine directly observed once per month. A standard WHO multibacillary dose-pack is shown [Image F]; the instructions in English must be clarified for all healthcare staff and patients. WHO now recommends only 1 year of therapy for multibacillary cases [controversy discussed in Lancet. 2004 Apr 10;363(9416):1209-19], but some would treat those with high bacterial indices (4 to 6+) for the previously recommended 2 years due to higher relapse rates. In some developed countries like the USA, multibacillary disease would be treated with 100mg Dapsone, 600mg Rifampin and 50mg Clofazimine a day for 2 years. A myriad of treatment reactions can occur, the possibility of which should be informed and explained to the patient before starting treatment and a reference text should be consulted prior to initiation of therapy by anyone not familiar with these. With the initial episode, the patient had been treated with the WHO multibacillary drug regimen for 12 months. This patient presents 2 years after completing therapy with a recurrence of disease and the question arises if this is a paucibacillary relapse or a late Reversal Reaction (see below). The presentation 2 years after completing therapy, the 3 months of evolution of the skin lesions and the lack of signs of a reaction in the biopsy suggest a relapse, although it is difficult to differentiate from a late reversal reaction, and many experts suggest a short trial of steroids, which will improve the reversal reaction and aid in the differentiation. While waiting for the drugs and biopsy results, the patient developed swelling and increasing erythema of the skin lesions [Image G], without nerve involvement. A diagnosis of Type 1 Reversal Reaction was made and therapy started with Prednisone 40mg/day together with the WHO multibacillary drug regimen (to be continued for another 1 year). Though this patient presents with a relapse 2 years later, the overall relapse rate reported with this regimen (but for the previous duration of 24 months) is less than 1% in patients with low bacillary loads and there is virtually no resistance to this drug combination. She should increase her cell-mediated immunity against M. leprae as bacilli die and she should be cured with no residual deformity. So-called Type 1 or Reversal Reactions may occur in up to one-third of borderline patients. These are caused by increases in T-cell reactivity to M. leprae with infiltration of reactive CD4 cells into skin lesions and nerves. After treatment is completed, some patients may partially recover their skin sensation, but longstanding nerve damage is irreversible. |