 Research published in the journal Gut suggests that nutraceutical strategies restoring tryptophan metabolism and IPA levels may serve as both biomarkers and therapeutic approaches for diabetic retinopathy.Diabetic retinopathy is a common condition caused by diabetes in which blood vessels in the retina are damaged, leading to blurred vision and in some cases blindness.
Research published in the journal Gut suggests that nutraceutical strategies restoring tryptophan metabolism and IPA levels may serve as both biomarkers and therapeutic approaches for diabetic retinopathy.Diabetic retinopathy is a common condition caused by diabetes in which blood vessels in the retina are damaged, leading to blurred vision and in some cases blindness.
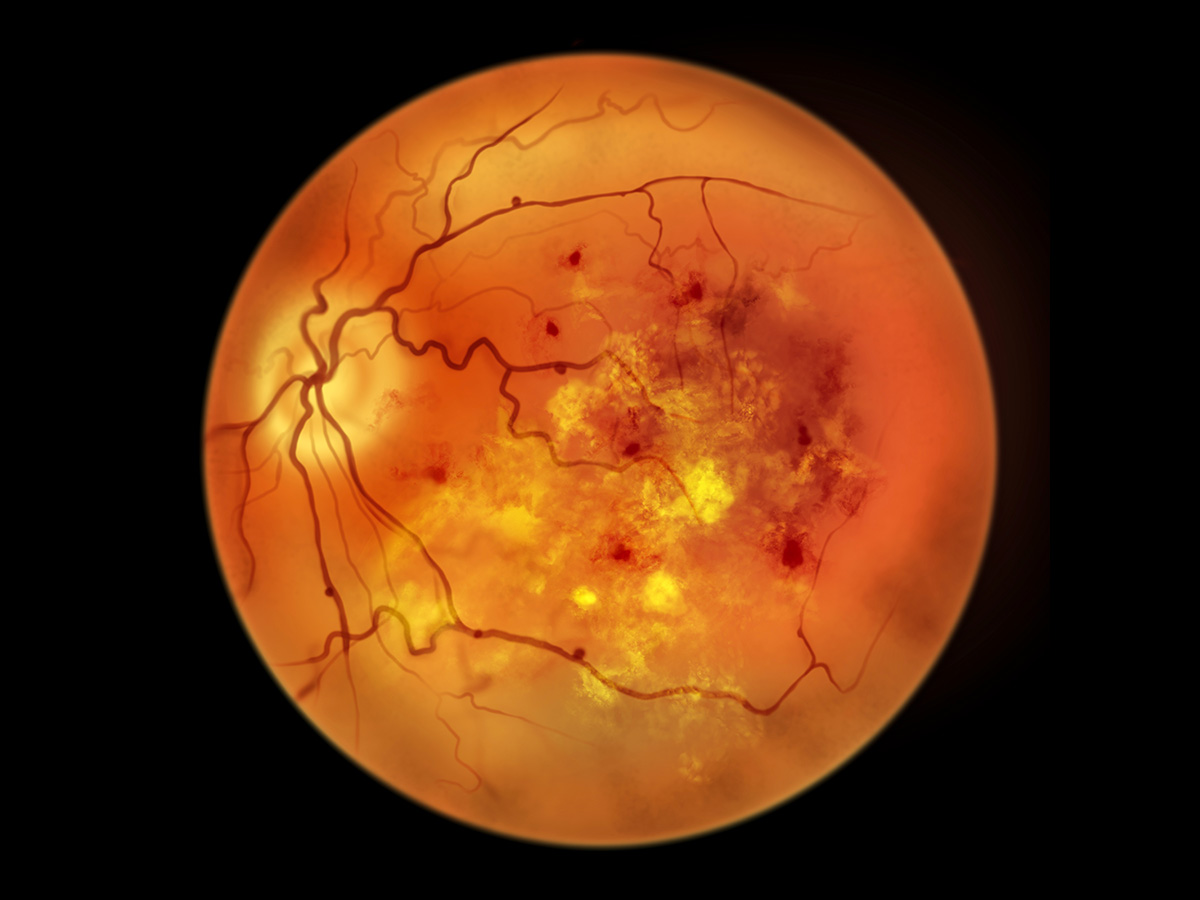
In research published in the journal Gut, investigators from the University of Alabama at Birmingham report that restoring tryptophan metabolism in the gut microbiome can prevent or reverse diabetic retinopathy, the leading cause of vision loss in working‑age adults with diabetes.
“By targeting how tryptophan is absorbed in the gut, we were able to correct dysbiosis, reinforce gut barrier integrity, normalize incretin signaling and ultimately protect the retina,” said Maria Bartolomeo Grant, M.D., senior author of the study and professor in the UAB Department of Ophthalmology and Visual Sciences. “These findings open a new avenue for microbiome‑guided metabolic therapies to prevent or delay vision loss in diabetes.”
Tryptophan is an essential amino acid that supports many vital biological functions, including maintaining a healthy gut microbiome. In Type 2 diabetes, however, the intestine loses its ability to efficiently absorb tryptophan. This impairment reduces the production of protective metabolites and increases inflammation, contributing to gut barrier breakdown and systemic disease progression.
Tryptophan is metabolized both by the human body and by gut bacteria. When tryptophan availability is reduced, bacterial metabolism shifts in harmful ways.
“A loss of tryptophan profoundly alters bacterial metabolism, leading to a marked reduction in the production of protective microbial metabolites,” said Ram Prasad, Ph.D., first author of the study and senior scientist in the UAB Department of Ophthalmology and Visual Sciences.
One of the key metabolites identified in the study is indole‑3‑propionic acid, or IPA, a gut‑derived compound that the investigators showed entering the bloodstream and accumulating in the retina, where it plays a protective role. Advanced imaging studies conducted at the Center for Advanced Spatial Biomolecule Research at the University of Florida, led by Borhane Ziani, Ph.D., Craig Vander Kooi, Ph.D., and Ramon Sun, Ph.D., revealed that IPA localizes to the posterior retina in healthy mouse models, providing the first direct evidence that beneficial bacterial metabolites reach and act within retinal tissue. In models of diabetic retinopathy, this metabolite is reduced.
The study demonstrated that in individuals with Type 2 diabetes the circulating IPA levels served as a biomarker for diabetic retinopathy. “Our findings suggest that restoring IPA to physiological levels has the potential to slow or counteract DR progression and may reduce broader diabetic complications,” Prasad said.
In addition to IPA, the researchers evaluated two complementary strategies to improve gut health: administration of Lactobacillus paracasei‑ACE2, a genetically engineered probiotic developed by Quihong Li, Ph.D., at the University of Florida, and supplementation with isoleucine‑tryptophan, a tryptophan‑containing dipeptide. Both approaches effectively corrected gut dysbiosis, enriched tryptophan‑metabolizing bacteria and significantly improved gut barrier integrity in experimental models of Type 2 diabetes.
“This study highlights the power of team science,” Grant said. “By combining expertise in microbiology, metabolism, ophthalmology and advanced imaging, we were able to uncover a gut–retina pathway with real potential for clinical translation.”