-
What is COMET?
The Lunaphore COMET™ platform is a fully automated liquid handler and microscope imaging scanner capable of performing sequential, multiplex, immunofluorescent staining and imaging samples adherent to a standard glass slide. The COMET™ system is fully customizable and capable of running 20 distinct staining conditions (i.e., optimization, mono-stains, dual-plexes) within a single run. The COMET system also operates with standard “off-the-shelf” antibodies, requiring NO conjugation. The transferability rate from applications optimized by hand or the BOND is very high. The COMET™ uses a proprietary microfluidic device that minimizes staining volumes and biochemistry dynamics, completing a single run in 24 hours. The platform has a four-slide capacity that maximally results in a 40-plex within 24 hours. Post-imaging, the slides can be used for multiple runs on the COMET™ or preserved for other applications (H&E, sequencing, inSitu Hybridization, thanks to the delicate elution buffer and low temperatures optimized for the sequential multiplexing).
For more information concerning the COMET™’s abilities and innovations please click here. -
Access to the services
The access to the services should be requested by sending a request to access to
This email address is being protected from spambots. You need JavaScript enabled to view it.
Please include the following information.
Full Name (First, Last):
Blazer ID:
Email:
PI full name.
The access is provided within 24 hours.
Once access to FBS has been provided please fill the COMET Sample Submission Form
-
Services - Antibody Bank
- All runs of the COMET™ system are required to use the FSCS COMET Antibody Bank. This invaluable resource accelerates our progress by allowing our community of users to build upon each other’s success and expertise.
- Currently, the bank represents panels reactive to human, mouse, rat, and canine tissue sections with markers spanning the tumor microenvironment, neurology, and immunology.
- This bank is a continually expanding resource and is guided by the experimental needs of our users.
- See the most up-to-date database here. Please contact us (
This email address is being protected from spambots. You need JavaScript enabled to view it. ) should you have questions.
-
Services - Assisted optimizations
- All COMET runs are Assisted.
- Optimization of markers takes on two forms: Mono/single antibody and Multiplex.
- Mono-optimizations.
- This is for new antibodies being added to the bank or testing of antibodies already existent in the bank but not yet tested on the desired tissue type or organism.
- The detailed optimizations are broken down into 3 “characterizations” as guided by Lunaphore. The FCSC core has procured normal C57Bl/6 and human tissue arrays for this initial characterization. The requesting investigator must provide tissue types not reflected in this array.
- Characterization 1: Does it work?
Best guess concentration (usually on the high side) on tissues where it should express. - Characterization 2: What’s the best concentration/exposure?
Approximately 3 concentrations to pick the best exposures and concentrations. - Characterization 3: How stable is the epitope?
Initial stain, then serial elutions (5-20x) followed by identical staining.
- Multiplex optimizations.
This is for the optimization of a full multiplex panel of antibodies in the bank and has proven to work on the tissue of interest on your specific tissue of interest. Every tissue stains differently; some markers may need to be tweaked on new samples. This is a check before a full cohort is run. When successful, the exact staining conditions will be used on the multiplex panel experiment. All data is saved and can be used as one of the samples.
-
Services - Multiplexing
This is for the optimization of a full multiplex panel of antibodies on the tissue of interest. Every tissue stains differently; some markers may need to be tweaked on new samples. This is a check before a full cohort is run. When successful, the exact staining conditions will be used on the multiplex panel experiment.
-
Sample Preparation
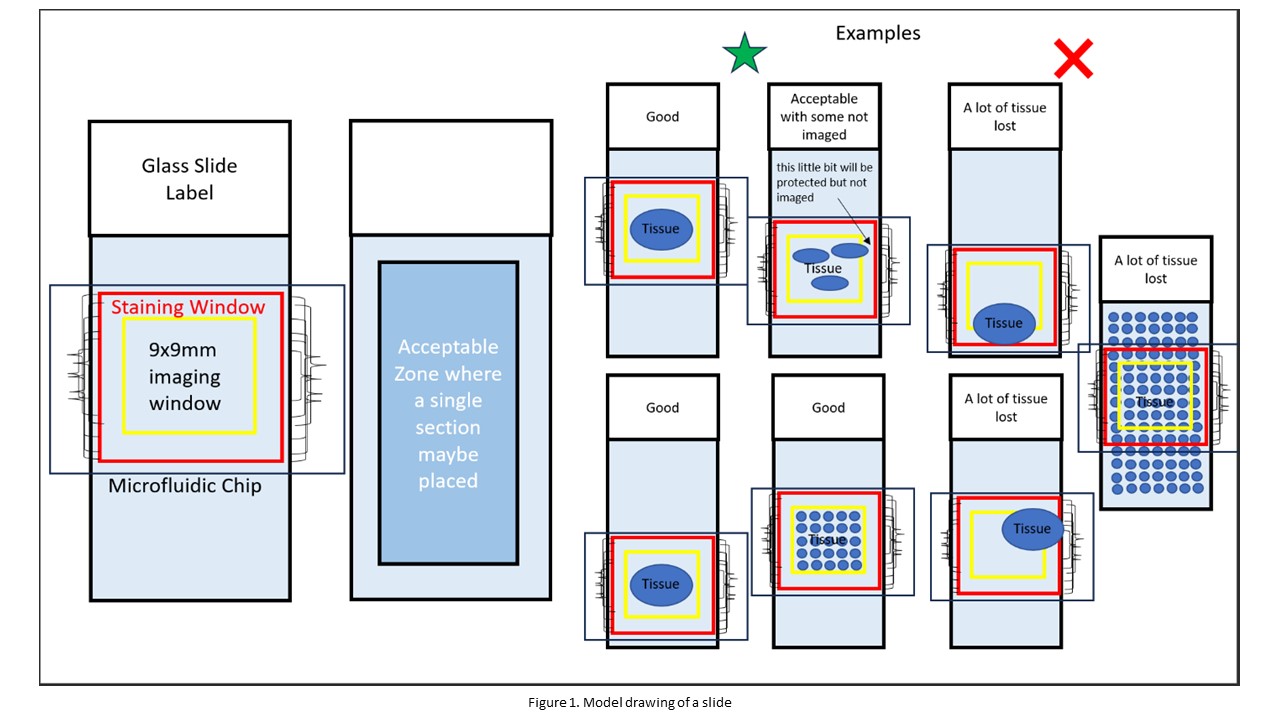
- The sample can be anything that can attach to a standard microscopic slide and fit within the staining and imaging window of the microfluidic chip. Some examples are tissue sections, FFPE, snap-frozen tissue, etc.
- Where you place your sample matters, a model drawing of a slide is provided in Figure 1. Anything within the blue box is an acceptable location for your sample. Thus, stars can be seen within the blue box as stars represent acceptable locations for your sample. In contrast, the triangles are located outside the blue box because they represent unacceptable locations for your sample.
- The red square in Figure 1 represents a metal wire within the chip. Anything within this metal wire is safe.
- The imaging window has a size of 9x9 mm. This imaging window will be directed toward the center of the slide. Thus, ensure that whatever you want to image is located at the center of your slide and can be contained within the 9x9 mm imaging window. Any part of your sample outside the 9x9 mm imaging window WILL NOT BE IMAGED. It is recommended that your sample be within 5x5 mm to ensure proper imaging. In Figure 1, the 9x9 imaging window is represented by the yellow square.
-
NIH Grant Document
Multiplex Immunofluorescence (mIF) Three research institutes at UAB (O’Neal Comprehensive and Cancer Center, Immunology Institute, and I4ward fund) have demonstrated continuing support in the form of physical infrastructure, personnel expertise (dedicated staff in a shared resource facility), and financial investment into the support of the proposed multiplex immunofluorescent (mIF) imaging studies. This combined investment has resulted in the recent purchase of the COMET multiplex imaging system, the establishment of an optimized antibody bank, and computational support for the analysis of mIF images in collaboration with the Research Computing and Cheaha Cluster teams. These endeavors are run at or below cost (i.e., supported by other grant mechanisms) to maximize the impact, accessibility, and successful use of these technological investments. Detailed training and workflows have been generated and are made available on-demand to all UAB and collaborating researchers. These investments and the collaborative interactions between the Biospecimen Bank (clinical samples) and Animal Core (pre-clinical samples) demonstrate an ideal environment for completing the proposed aims.
EQUIPMENT
Lunaphore COMET Multiplexing System.
Located within the Flow Cytometry and Single Cell Shared Resource on the UAB campus in the Shelby Research building. The COMET system is a self-contained liquid handler and microscopic image scanner in one fully modifiable program to optimize and validate three fluorophores each cycle. An optimized antibody bank and a BioGenex EZ-Retriever antigen retriever system support the COMET system. The fully trained core staff supervises all reagents, antibodies, protocol optimizations, and equipment operations.
Visiopharm Image Analysis Software.
UAB investigators and core facilities have access to several seats of the full research license of Visiopharm analysis software. Access to 75TB of storage for each research faculty within the Research Computing core is provided to store multiplex scanned images for analysis. These systems come with several hours of detailed user support and training each month and access to on-demand, online overview training for all future and current users, supported by Dr. Carstens.
-

Flow Cytometry and Single Cell Core Facility -

Flow Cytometry and Single Cell Core Facility -

Flow Cytometry and Single Cell Core Facility -

Flow Cytometry and Single Cell Core Facility -

Flow Cytometry and Single Cell Core Facility -

Flow Cytometry and Single Cell Core Facility -
Flow Cytometry and Single Cell Core Facility