Dr. Shama Ahmad’s laboratory is focused on investigating mechanisms of toxic inhaled environmental or industrial chemical-induced pulmonary and/or cardiac toxicity, with the aim to establish agents/methods to mitigate the harmful effects of these agents.
Education
M.S., Lucknow University, Lucknow, India.
M.Phil., Institute of Biotechnology, A.M. University
Ph.D., Institute of Biotechnology, A. M. University
Contact
Office Location
BMR II, 322
Email
shamaahmad@uabmc.edu
Phone
205-975-9029
Active Research Projects
Acute airway injury and repair
Objective: Aberrant repair due to inefficient stem/progenitor cell function is a known cause of pathogenesis of acute and chronic airway diseases. Our goal is to restore/improve endogenous stem cell function post injury for efficient repair and limit airway disease pathogenesis.
Rationale: The extent of airway injury determines survival during exposure to toxic inhaled chemicals. In animal models, exposure to reactive gases such as Cl2 can cause dose-dependent early lethality with later complications such as infections developing from loss of normal airway epithelial barrier function. Injury to the epithelium of conducting airways is a common feature of human illness and animal models of gaseous or smoke exposures. Airway epithelial basal cells (BCs) function as stem/progenitor cells to restore other airway cell types following injury. Exposure to Cl2, causes depletion of BCs. Abnormal epithelial repair due to experimental (conditional depletion) or natural loss of BCs/cell function contributes to pathogenesis of many chronic airway diseases. BCs express tissue factor (TF), a cofactor in the clotting cascade, that may also be used as a marker to isolate them. However, the significance of BC surface TF expression and its role in airway injury repair is unknown. We have demonstrated that a high TF-expressing BC population exhibiting enhanced proliferative and clone-forming capacity can be derived from human airway epithelium. TF-expressing BCs constitute a major and consistent population of epithelial cells purified from a range of donors. TF activity on the surface of these BCs promotes cell attachment, survival and proliferation via protease-activated receptors, PARs. The role of TF in supporting BC functions is novel and could have important implications for both normal healing and pathological airway remodeling.
Brief description of methods: We utilize murine and rodent models of halogen gas exposure to study airway injury. We measure various physiological parameters for respiratory functions. We utilize in vitro and ex vivo assays for measuring progenitor cell functions. We also perform biochemical assays to analyze molecular signaling mechanisms.
Impact on human health: Acute airway injury, a subset of ARDS, is a devastating condition caused by direct exposure to toxic inhaled chemicals (TICs), smoke inhalation and acid aspiration. For all forms of ARDS, since treatment is still limited to supportive measures, mortality remains high (approximately 74,500 deaths/yr). Our research aimed at improving the basal cell function to enhance endogenous repair seeks to provide novel treatments for respiratory diseases.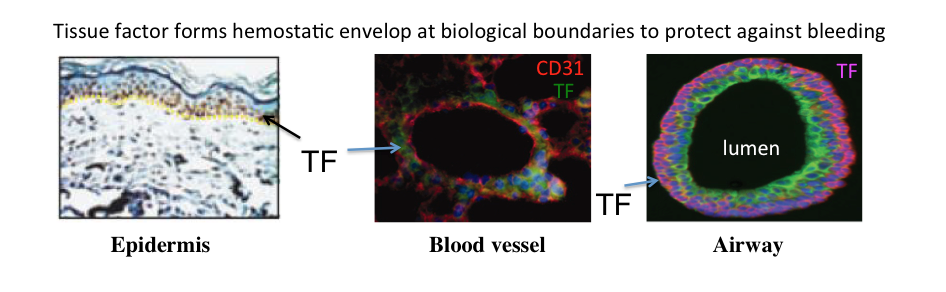
Cardiopulmonary impact of inhaled halogen gases
Objective: Halogen gases such as chlorine and bromine are highly reactive, corrosive ‘inhalational’ threat agent that can spread both as liquid and as fumes. Inhalation of chlorine/bromine causes extensive injury to the lungs and the heart. Our goals are to identify the biochemical and molecular mechanisms responsible for these injuries and develop effective countermeasures.
Rationale: Accidental leaks from manufacturing plants are common and large groups of people may be exposed to high halogen (Cl2/Br2) concentrations. Use of halogen gases as chemical weapons is also on the rise. Victims of accidental bromine exposure experience respiratory distress, cardiac arrest and circulatory collapse. Studies evaluating acute and chronic sequelae of Br2 exposure are scant and treatment remains symptomatic as no effective countermeasures exist. Our studies have established that the heart is severely injured in animals that survive high dose halogen (Cl2/Br2) inhalation. Brominated reactants/lipids reach the heart along with oxygenated blood. These reactants inactivate important calcium pumps that regulate the heartbeats. Inactivity of calcium pumps causes calcium accumulation or “calcium overload” in the heart cells. Calcium overload is a serious problem and can lead to sudden cardiac death. Increased calcium also activates destructive proteins, the calpains, that destroy cardiac ultrastructure.
Brief description of the methods: We utilize murine and rodent models including general and conditional SERCA knockout mice and rats for halogen exposures and evaluate the physiological impact by pulse oximetry, arterial blood gas measurement, echocardiography and telemetry. We also investigate the tissues (lung and heart) for protein and RNA expression.
Impact on human health: The outcome from this project will identify effective antidotes for halogen/bromine toxicity and enhance readiness for emergencies arising from accidental or intentional exposures.
 Tenured Professor
Tenured Professor