Research Information
A number of experimental techniques that involve measurements in CF patients are crucial to advancing CF science – including our understanding of basic aspects of disease pathogenesis, characterization of novel therapeutics directed towards the CF ion transport defect, and the evaluation and rapidly evolving role of CFTR modulators in human subjects, including a variety of effects on the endocrine and digestive system.The Clinical and Translational Core, established at our Center in 1999, provides necessary resources and expertise to provide these capabilities for a wide variety of CF scientists, significantly improving the ability to identify and translate CF scientific advances to new therapeutics.
Core Function # 1: Design and Conduct in vivo Measurements of CFTR Activity in Human Subjects.
Measures of ion transport in human subjects are diagnostic of the CF defect, and can also be used to monitor the response to agents that alter ion transport pathways, including novel genetic therapies intended to restore CFTR activity. The Core can conduct assays of CFTR activity in human subjects, including:- Nasal potential difference (NPD)
- Lower airway potential difference (LAPD) by agar catheter - also adapted for measurement of sinus PD
- Sweat chloride analysis (in real time or in batch), sweat secretion by evaporimetr
- Rectal intestinal current measurements
- Endoscopic CFTR function by short circuit current in vivo and in situ – in development for use in the gut and airways of humans, addressing gaps posed by PD measurements
The Core also provides study design, infrastructure, and regulatory support for observational and interventional clinical studies requiring endpoints involving CFTR activity, including measures of PD, or alternative methods (e.g., sweat testing, etc.). Expertise provided includes:
- Generalized study design
- Sample size estimates
- Source document production
- Alteration of PD apparatus for specific purposes
- Practical considerations regarding the conduct of complex studies
- Assistance with assuring the protection of human subjects and with timely preparation, submission, and tracking of IRB documents and MTA submissions
Core Function # 2: Conduct Cardinal Measures of Airway Epithelial Cell Function in vivo.
Epithelial host defense relies on an intact mucosal barrier. In the CF lung, abnormal mucus, alterations in airway surface liquid (ASL), and delayed mucociliary transport (MCT) compromise host defense, and are key targets for novel therapeutic interventions and the evaluation of emerging concepts in CF pathophysiology. Abnormal mucus also characterizes the GI tract.To address this, the Core provides expertise in two high impact, well utilized, and complementary techniques for assessing key aspects of mucociliary clearance in vivo:
- Micro-Optical Coherence Tomography (μOCT) imaging in vivo, along with image acquisition, data analysis, and validation
- Whole-lung mucociliary clearance (MCC) using clearance of Tc99 particles
μOCT is an innovative technique that provides real-time imaging of airway epithelia, and can quantitatively determine ASL/periciliary layer (PCL) depths, ciliary beat frequency (CBF), and MCT of living cells. It provides an ideal system for secondary characterization of agents thought to alter ion transport, ciliary beating, or physical properties of the mucus while also providing new insight towards CF pathophysiology in the airways.
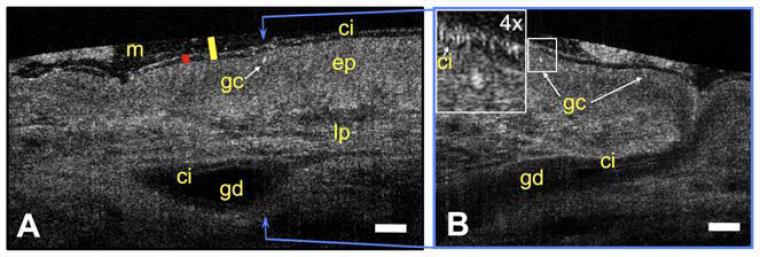
μOCT functional anatomy of human trachea
Core Function # 3: Support The Execution of CF Clinical Studies
Our Center has been highly active in the design and conduct of CF and CFTR-related clinical trials, and the Core provides the following requisite expertise and support to continue this momentum:- Clinical trial design and regulatory support - includes CF-related IND and/or IDE applications, coordinated with the UAB Center for Clinical and Translational Science
- Nutritional outcome measures - bone mineral densitometry via dual-energy X-ray absorptiometry, indirect calorimetry, bioelectrical impedance analysis, oral glucose tolerance testing, continuous glucose monitoring (offered in concert with the UAB Nutrition Obesity Research Center and Diabetes Centers)
- Spirometry
- Lung clearance index (LCI)
- Biospecimen collection
- Intestinal, nasal, and skin cell procurement
- Sputum induction and processing - spontaneously expectorated and induced sputum, with capability to estimate mucus hydration by solid content and biophysical properties of mucus by cone and plate rheometry
- Tissue procurement – lung and GI tissue
