Research Information
Established in 2002, the Animal and Preclinical Models Core serves to provide specialized capabilities for developing and characterizing numerous CF animal models relevant to CF pathogenesis and treatment. The three key functions of the Core are listed below:
Core Function # 1 : Breed, Genotype, and Distribute Diverse CF Animal Models
-
CF Knockout Mice
These CF mouse models produce no functional Cftr mRNA or protein, and are particularly useful to stufy consequences of the electrophysiological defect.
The North Carolina knockout mouse (Cftrtm1Unc) line: Contain a Neo cassette in exon 10 of the murine Cftr gene and exhibit a severe phenotype that includes early intestinal obstructive and perforation lesions similar to meconium ileus/distal intestinal obstruction syndrome (DIOS) in CF patients.
The Cambridge knockout mouse (Cftrtm1Cam) line: Useful for studies of nonsense suppression agents because exon 10 of the mouse Cftr gene was disrupted with the HPRT gene, rather than a Neo cassette. Bi-transgenic mice developed using this knockout line that are available include:
- FABP-hCFTR mice: Express a wild type human CFTR transgene from the intestine-specific FABP promoter on a Cftrtm1Cam background, facilitating studies when intestinal phenotype is not required.
- FABP-hCFTR-G542X mice: Express a human CFTR-G542X transgene from the intestine-specific FABP promoter on the Cftrtm1Cam
- FABP-hCFTR-W1282X mice: Express a human CFTR-W1282X transgene from the intestine-specific FABP promoter on the Cftrtm1Cam knockout background.
Mouse lines carrying a conditional null allele of Cftr (Cftr10fl) or a constitutive Cftr null (CftrD10): Allow the investigation of Cftr function in specific tissues or in different developmental time points.
-
CF Mice Harboring Clinically Relevant Mutations
CF mice in this category have been engineered to express altered CFTR mRNA and protein that correspond to mutations found in CF patients. Depending on the class of mutation introduced, animals may have distinct phenotypes and/or function in comparison to CTFR knockout mice.
The Erasmus Cftr-F508del line (mCftrF508del Erasmus): Cftr mRNA levels are normal and the strain shows many functional characteristics of the human F508del allele.
The Cftr-G551D mouse line (CftrG551D): Constructed by B Wainwright’s group when he was located at the University of Queensland, Australia. As Dr. Wainwright has since left Queensland and is no longer able to provide it, we acquired this line and now provide it to the broad CF community as an important resource.
The Cftr-G542X knock-in mouse line (CftrG542X): Useful to examine nonsense suppression therapies that target either nonsense suppression or nonsense mediated decay (NMD) inhibition because the G542X mutation was introduced into the mouse Cftr locus and thus the transcript is spliced normally and is subject to NMD.
-
CF Rat Models
CF null (CFTR-/-) rat: The first CF rat model, CFTRtm1sage, was designed in collaboration with SAGE (now Envigo) in 2011 and provided to UAB for analysis and distribution to the CF community.
The model has been characterized through age 6 months, and fills an important niche as CF mice have more limited value in modeling airway disease beyond the electrophysiological phenotype, whereas CF pigs and ferrets are highly informative, but their poor survival, size, complexity, and cost inhibit extensive use or evaluation in vivo.
Core Function # 2: Generate and Procure Relevant CF Animal Models
The Core is aggressive in generating new CF animal models (e.g., novel mice or rats), or procuring animals, live tissues, or samples from CF ferrets, rabbits, and pigs. Provision of these CF animal models and tissues is a key function of the Core.
This includes:
• Development of new CF relevant mouse models (eg, Rpl12, dnUPF1, SFTPC-Muc5b, Scgb1a1-Muc5b)
• Assistance in the procurement and analysis of CF ferret models – includes G551D ferrets (through an existing Material Transfer Agreement with Marshall Farms and University of Iowa)
Core Function # 3: Conduct CFTR Functional Assays and Additional Endpoints
-
Assays
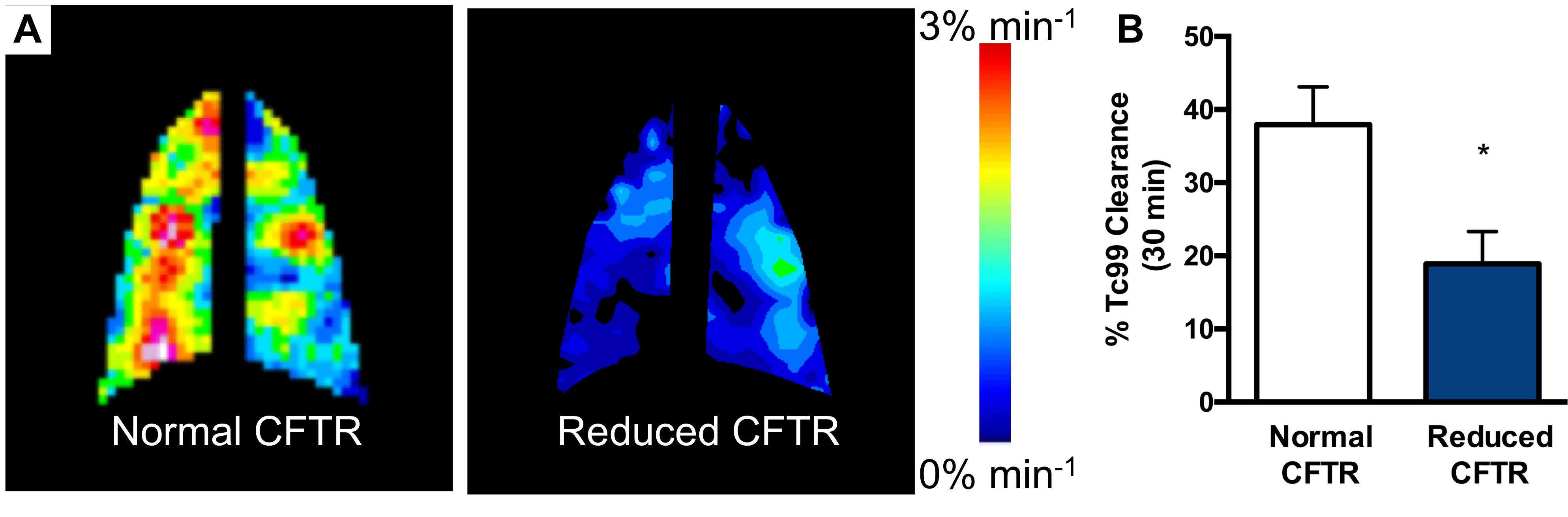 Reduced in vivo MCC in rats with diminished CFTR activity.
Reduced in vivo MCC in rats with diminished CFTR activity.- In vivo CFTR electrophysiology (Nasal and Lower Airway potential difference). The Core operates a potential difference (PD) apparatus dedicated and optimized for the measurement of Nasal PD (NPD) in mouse, rat, rabbit, and ferret nares, and lower airway PD (LAPD) in rats and ferrets.
- Short circuit current (Isc) measurements of excised trachea and intestine. Short-circuit current (Isc) attributable to CFTR activity can be readily determined in trachea or intestine explants in Ussing chambers.
- In vivo and ex vivo micro-Optical Coherence Tomography (µOCT) imaging / in vivo microscopy. µOCT supports simultaneous acquisition of data regarding airway surface liquid height, ciliary beat frequency, mucociliary transport, and mucus viscosity from the surface of airway mucosa. Such in vivo data can be supplemented with in vivo microscopy capabilities of the Core, as well as with µOCT imaging of freshly harvested rat and ferret trachea with or without further experimental exposures ex vivo in conjunction with the Functional Assay Core.
- In vivo mucociliary clearance (MCC) assay. In collaboration with the UAB Small Animal Imaging Core facility, the Core can employ a radiographic MCC assay using 99mTc-DTPA or Sulfur Colloid to estimate MCC. This method has been successfully employed to estimate MCC in mice, rats, and ferrets
- 6-Voxel resolution computed tomography. The Core houses a state-of-the-art preclinical CT scanner (U-CTUHR) with dedicated beds and automated protocols for mice, rats, rabbits, and ferret imaging that can support in vivo settings capable of generating very fast, low-dose acquisition of soft and hard lung tissues structures at an extremely high resolution of 6 micrometer voxel size in vivo.
- Measurement of lung function (Flexivent). Obstructed airways are a characteristic clinical feature in CF patients. Lung functions in diseased ferrets and rats (and mice if needed) can be estimated using forced impulse oscillometry
- Abdominal ultrasound. Using abdominal ultrasound, we can detect the presence of intestinal obstruction even before the rat shows clinical signs of distress. This is a non-invasive and repeatable survival procedure that is possible in even young (4-week old) rats. Targeted measurements include bowel wall thickness, luminal diameter, and luminal thickness to determine the incidence and severity of distal intestinal obstruction syndrome (DIOS), an outcome of interest with novel mucolytics..
- Cough monitoring. Cough frequency is central to the clinical diagnosis of chronic bronchitis in CF patients, and the Core can measure this metric in conscious ferrets and rabbits. Free-moving animals are monitored for frequency of coughing using a state-of-the-art system combining simultaneous video, acoustic, and plethysmography measures.
- Glandular CFTR assay. The importance of glandular CFTR activity is supported by the high sensitivity of sweat gland assays in predicting disease severity and drug trial outcomes. The Core has advanced a reliable glandular CFTR activity assay by measuring salivary secretion rate in mice and rats. Similar to human sweat chloride testing, adrenergic-induced salivary secretion is attributed to alterations in CFTR ion transport, and is highly diminished in CF animals.
- Electroporation-mediated gene manipulation. To address the role of single gene alterations in airway disease pathogenesis and to validate the effectiveness of emerging pharmacologic targets, our Center has engaged electroporation (EP)-mediated gene delivery to alter the expression of genes of interest. We have adapted this technique for use in the lungs of mice, rats, and ferrets, and the Core can help implement this experimental tool to affect expression of CFTR or other targets.
-
Specimen Collection and Analysis
 The Core provides the expertise needed to conduct thorough histopathologic analyses of airway structure and other organ defects relevant to CF.
The Core provides the expertise needed to conduct thorough histopathologic analyses of airway structure and other organ defects relevant to CF.-
- Miniaturized bronchoscopy for longitudinal lung sampling. Our CF Center has acquired an innovative small animal bronchoscope (Polydiagnost, Germany) that enables collection of bronchoalveolar lavage (BAL) samples and supports delivery of substances to specified lung lobes. The Core supports this miniaturized video-bronchoscope for various lung research applications in mice, rats, and ferrets.
-
Standardized Anesthesia, Physiologic Monitoring, and Intubation for Exposure and Assessment Procedures
The Core provides the expertise and resources for design and execution of anesthetic regimens that are vital to achieving the level of sedation required for experimental success and to ensure animal welfare and survival for repeat measures. Further, the Core offers expertise in airway intubation to provide access to lungs in live animals for imaging, specimen collection, and uniform delivery of various experimental agents directly into the lungs.
-
- Inhalational Delivery. Localized delivery to lungs is critical for many respiratory experimental endpoints, and Core B provides CF Center investigators with the access, resources, and expertise needed for administering aerosolized diagnostic agents (e.g., Tc99-based radiographic labels for MCC, CT-contrast agents), pharmacologic tools (e.g., interventional drugs or probes), as well as biologics (such as infectious agents) or gene repair agents (such as recombinant oligonucleotides and mRNA therapeutics), to the lungs of mice, rats, rabbits, and ferrets using a variety of aerosolizing devices.
Contact Information
Core users are associated both within and beyond the CF Research Center; there is no limitation on who may access the Core. For additional information regarding resources and services, please contact:

