 |
Gorgas Case 2017-04 |
 |
|
The following patient was admitted to the Pediatric Intensive Care Unit at the Cayetano Heredia Hospital in Lima, Peru.
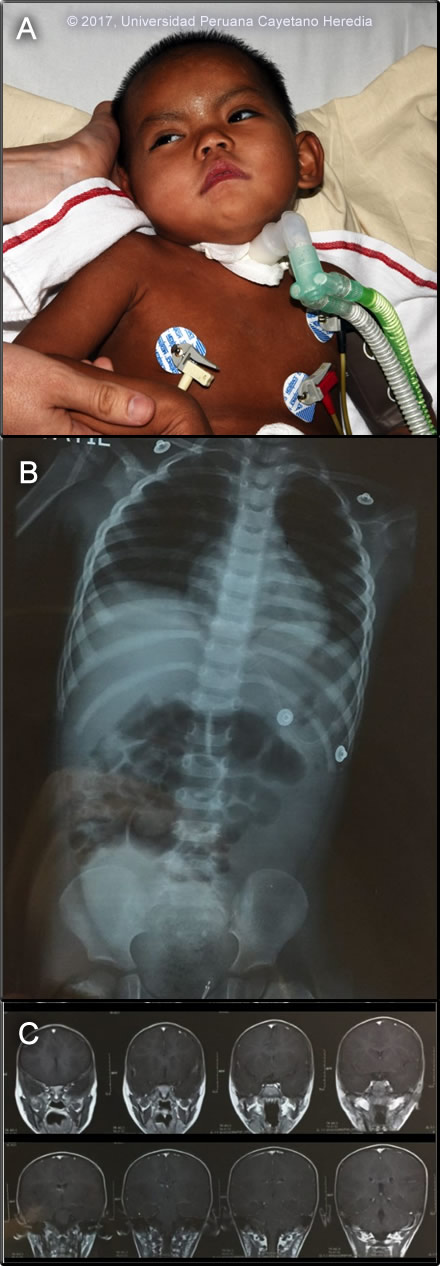
 History: A 2.5 year-old female presented to a local hospital with a 6-day history of fever followed by neurologic symptoms. Despite a negative thick smear, a community healthcare team started empiric treatment for malaria with cloroquine and primaquine on the third day of non-specific fever that had failed to respond to anti-pyretics. On the third day of illness, ataxia and trembling of the lower extremities began and later progressed to the upper extremities. Lower extremity weakness then progressed to an inability to stand unassisted. On day 6, anorexia and vomiting began until on day 7 when she began to have difficulty swallowing, increased salivation and stuttering speech. On day 8 she was still febrile, became hypoactive, unable to follow instructions, and finally somnolent (possible status epilepticus) before transfer to our hospital. No prior medical history.
Epidemiology: Born and raised in an agricultural family in the Mayapo community, province of La Convencion in the jungle of Cuzco department. She had no history of malaria, was unvaccinated for yellow fever, and had no known exposure to TB. She had received 1 ml. doses of tissue culture rabies vaccine 11 and 4 days prior to the onset of fever as part of a pre-exposure vaccination campaign in her community following some earlier encephalitic fatalities. Physical Examination (arrival at our ER): HR 108, RR 32, BP 89/33 mmHg, T 37ºC, SatO2 95% Acutely ill patient. Skin: no rash. Chest: diffuse rhonchi bilaterally. CV: regular, tachycardic, multifocal murmur II/VI. Abdomen: bowel sounds present, no tenderness, no pain. Neurologic: Glasgow 12/15, somnolent, not following verbal commands. Opens her eyes after painful stimuli and localizes pain. Hypotonic, muscle strength not assessed. Normal reflexes, Babinski negative, no clonus, Hoffman negative. No meningeal signs. Image A shows the child in the ICU. Laboratory Examination: WBC 19.9 (2 bands, 73 segmented neutrophils, 3 eos, 7 monos, 15 lymphs), Hb 7.6 g/L, platelet count 494 000 /uL. Glucose 85 mg/dL, BUN 9 mg/dL, creatinine 0.3 mg/dL. Total protein 6.6 g/dL, albumin 3.2 g/dL. Total bilirubin 0.4 mg/dL AST 36 U/L, ALT 25 U/L, AP 166 U/L. CXR and MRI are shown in Image B and Image C. CSF: WBC 79 cells/hpf (90% lymphocytes), RBC 25 cells/hpf, glucose 51 mg/dL, protein 91 mg/dL. Malaria smear negative.
UPCH Case Editors: Carlos Seas, Clinical Course Coordinator / Sofia Zavala, Associate Coordinator UAB Case Editor: David O. Freedman, Course Director Emeritus / German Henostroza, Course Director |
|
Diagnosis: Paralytic rabies.
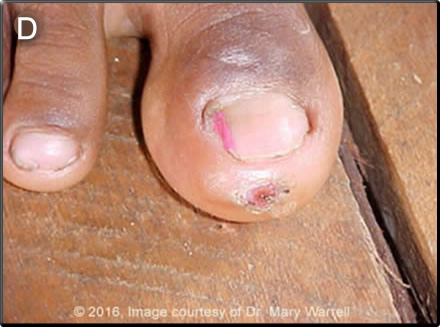
 Discussion: Giemsa stain of blood smear for Bartonella: negative. IgM ELISA for dengue, yellow fever, Oropuche, Mayaro, Venezuelan Equine Encephalitis: negative. PCR for rabies on nuchal skin biopsy and saliva: positive. Rabies neutralizing antibodies in serum: 2.2 IU/ml and neutralizing antibodies in CSF: 0.5 IU/ml. In general, in studies on dog rabies in Africa, rabies can be most efficiently diagnosed with PCR of saliva on 3 samples; if any are positive rabies is confirmed and will quickly guide the best course of action. A number of abnormalities on brain CT and MRI have been described in rabies but none is diagnostic and this patient had only non-specific cerebral edema. Further detailed history determined that a mass pre-exposure vaccination campaign had been started after two cases of bat rabies were confirmed in her community. The parents reported that coincidentally, the patient had been bitten by a bat on the scalp at night despite using a mosquito net. As part of the mass vaccination campaign, she received a dose of rabies vaccine (tissue culture vaccine) two days after the bite and a second dose 7 days later, these two doses were actually intended for pre exposure prophylaxis. Furious rabies is the more common clinical presentation of rabies in humans but this case, as with almost all cases transmitted by vampire bats, represents the less distinctive paralytic form of rabies disease. After prodromal symptoms, which include fever, headache and local paresthesias (not reported in this patient), an ascending flaccid paralysis with pain and fasciculation in the affected muscles and mild sensory disturbance ensue. Symptoms tend to begin around the site of the bite in the affected limb. A careful search for the telltale bite should be undertaken (example from another patient in Image D). Paraplegia and sphincter involvement then develop and the terminal event is usually a fatal paralysis of the swallowing and respiratory muscles. Hydrophobia, common in furious rabies, is very rare. Survival from onset of disease may be several weeks if no complications ensue. Other possible diagnoses include HSV encephalitis and VEE, plus many other arboviral encephalomyelitides e.g Oropouche. Guillain Barré (ascending paralysis) and severe tetanus with autonomic instability may present with similar peripheral symptoms. Rabies is caused by RNA viruses of the genus Lyssavirus. Classic Rabies virus (species I) is the most common of the 6 viral species in the that cause an identical rabies-like illness, and it is responsible for more than 55,000 human deaths annually. Outside of the Americas, most human cases are associated with domestic dog bites and exposures. Rabies virus is transmitted in the saliva inoculated by the bite of an infected mammal. In the Americas, bats and carnivores are the major reservoirs and multiple insectivorous bat species transmit the virus to humans in the United States. In Latin America, rabies is transmitted by the three species of vampire bat, especially Desmodus rotundus which are confined to middle/south America and some Caribbean islands. Wide circulation of rabies among vampire bats, which are efficient transmitters, throughout their geographic range occurs throughout Latin America has resulted in outbreaks, first confirmed in Trinidad (1925-32), and in a number of Latin American countries including Ecuador, Venezuela, Brazil as well as Peru. A variety of bat lyssaviruses in the genus circulate widely in bats in other parts of the world and have been associated with a relatively small numbers of cases of clinical disease identical to rabies. In Peru, urban dog rabies has decreased dramatically since 1995 thanks to mass vaccination programmes notably in Lima and Callao. Most recently [INS 24/02/2017], canine rabies has recurred in Puno and Arequipa., and in 2014 the 14 cases in canines were restricted to Puno, Puerto Maldonado, and in 2015 an emergence of 10 canine cases occurred in Arequipa with 1 human case. A number of significant outbreaks of rabies in humans associated with vampire bat rabies have occurred throughout the jungle regions of the country over the past several decades. Four human cases of rabies were reported in Peru in 2015, one was urban rabies, but 15 cases were associated with vampire bat bites in 2016. During epizootics of vampire bat rabies, cows (a preferential food source for vampire bats) become infected first and dying cattle with evidence of vampire bat bites often herald human outbreaks. Bat bites, most of which are un-noticed, unlike in our present case, are common in indigenous regions. Recently, it has been noted that 11% of healthy individuals randomly tested in Loreto had neutralizing antibodies to rabies (Am. J. Trop. Med. Hyg., 87(2), 206-215, 2012) indicating that sub-clinical and/or non-fatal infection with lyssaviruses is possible. Risk factors for bat exposure included age less than or equal to 25 years and owning animals that had been bitten by bats. In managing patients with confirmed rabies encephalitis, the doctor’s primary responsibility is to palliate their terror, pain and distress. Intensive care might be considered if an American bat virus is thought to be involved (they may be less virulent), some vaccine was given before onset of encephalitis, rabies neutralising antibody was detectable early, and intensive care facilities are available. All these conditions were fulfilled in this patient who has now survived for >70 days after onset of symptoms but remains with serious neurologic impairment. The controversial Milwaukee protocol (frequently revised/updated) comprises a variety of interventions aimed at controlling dysautonomia, vasospasm and other commonly observed complications. It has no proven advantage over standard intensive care (J Neurol Sci 2014: 339: 5–7; Can J Neurol Sci 2016: 43: 44–51). Neither rabies vaccine nor rabies immune globulin (not available in Peru) is of benefit after the onset of clinical rabies. |