 |
Gorgas Case 2020-01 |
 |
|
The Gorgas Courses in Clinical Tropical Medicine are given at the Tropical Medicine Institute at Cayetano Heredia University in Lima, Perú. For the 20th consecutive year, we are pleased to share interesting cases seen by the participants that week during the February/March course offerings. Presently the 9-week Gorgas Course in Clinical Tropical Medicine is in session. New cases will be sent by e-mail every Tuesday/Wednesday for the next 9 weeks. Each case includes a brief history and digital images pertinent to the case. A link to the actual diagnosis and a brief discussion follow.
Carlos Seas and German Henostroza
Course Directors |
|
The following patient was seen in the outpatient clinic of the Infectious Diseases Department of the Hospital Cayetano Heredia during the Gorgas Diploma Course.
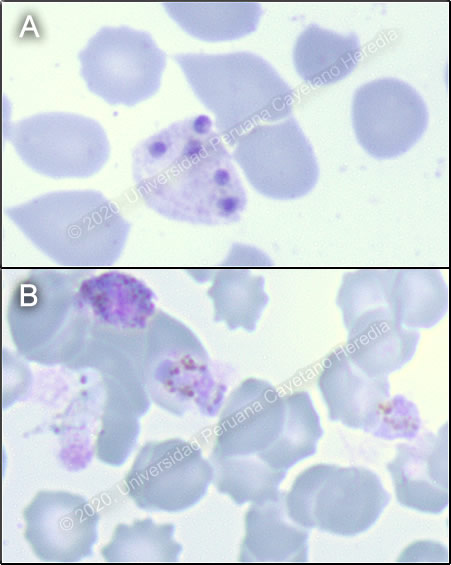 History: A 53-year-old male patient presented to the ER with a 6-day history of headache, myalgia, sweats and fever (cold shivering, then felt hot and exhausted) at the same time every night. He noticed slight jaundice in the face and sclerae, as well as dark urine, on the third day of illness. Additionally, he presented myalgia, predominantly in the lower extremities, which intensified causing difficulty walking. On the day of admission, he presented nausea and vomiting. Epidemiology: He is originally from Venezuela but migrated to Lima two years ago. One month prior to symptom onset, he was working as a construction worker in Tumbes, in northern Peru. Symptoms started on the last day of the trip. He denies any previous illnesses or surgeries. Unsure if has received yellow fever vaccine. Denies exposure to freshwater. Reports regularly eating from street vendors. Denies risk factors for sexually transmitted infections. Physical Examination: BP: 80/60 mmHg; RR: 26; HR: 75; T: Afebrile. Patient in no acute distress. Marked pallor, dry mucous membranes, no mucosal petechiae, no jaundice, normal capillary refill. No rash or petechiae. No lymphadenopathy. Pulmonary and cardiovascular exams within normal limits. Abdomen had diminished bowel sounds, distended, liver was palpable 2cm below ribs, spleen was not palpable, tenderness on palpation in left hemiabdomen. Patient was awake, oriented, Glasgow Coma Scale 15/15, no focal deficits, no meningeal signs. Imaging Studies: Normal CXR on admission. Laboratory: Hb:12.6 g/dL; Hct. 26%; WBC 4.01 (bands: 8%, neutrophils: 64.1%, eosinophils: 11%, basophils: 0.2%, monocytes: 3%, lymphocytes: 13%); Platelets: 28 000. Gluc: 148 mg/dL, Urea: 44 mg/dL, Creat: 1.5 mg/dL, Na: 139 mEq/L, K: 4.44 mEq/L, Cl: 102 mEq/L, Total bilirubin 2.6 mg/dL, Indirect bilirubin 1.9 mg/dL, Alk phos 144, AST 101, ALT 94, GGT 124, CRP 74.29, HIV non-reactive. Urinalysis was unremarkable. Arterial blood gases: pH 7.42, PCO2 31, PO2 100, HCO3 22, Lactate 2.3 Thin smear is presented in Images A and B. |
|
Diagnosis: Malaria - mixed infection with Plasmodium vivax and Plasmodium falciparum 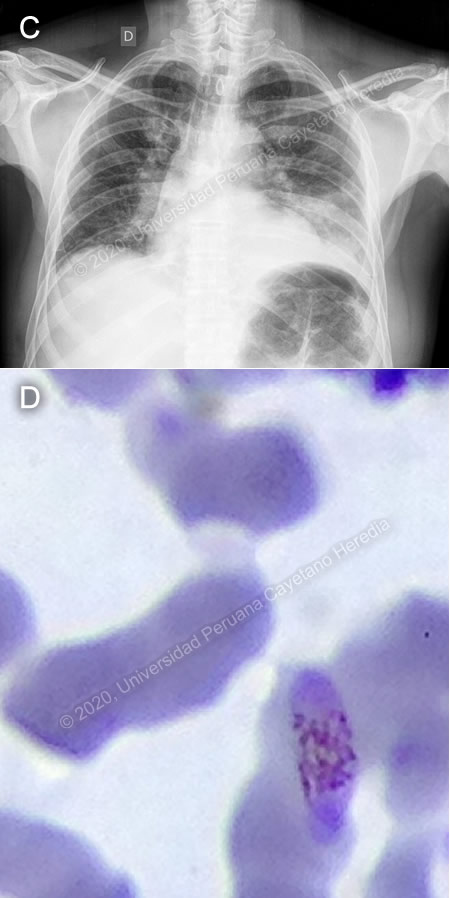
 Discussion: P. vivax schizonts and gametocytes (Images A, B) were found on initial thick smear. There was concern for severe malaria due to the patient’s presentation, with hypotension, elevation of bilirubin, and altered renal function. One day later, the patient reported shortness of breath, with oxygen saturation to 77%, requiring supplemental oxygen. Chest X-ray showed a new consolidation in right base (Image C). Then, new slides prepared from the same blood samples collected on admission revealed P. falciparum gametocytes (Image D). "An “Effective” (Hangzhou AllTest Biotech) rapid antigen detection test was positive for P. vivax but negative for P. falciparum. This test contains P. vivax anti-HRP-II and anti-pLDH antibodies and P. falciparum anti-HRP-II antibodies. This rapid test has a reported sensitivity of 95% and specificity of 99% for P. vivax and P. falciparum, when compared to microscopy [Cochrane Database of Systematic Reviews 2014, doi: 10.1002/14651858.CD011431] Discrepancy between the microscopy findings and rapid test results for our patient may be explained by absence of HRP proteins due to pfhrp2 or pfhrp3 deletions in some P. falciparum parasites. Peru has the highest documented levels of such deletions [PloS One 2010, doi: 10.1371/journal.pone.0008091]. Genetic analysis conducted on 188 P. falciparum samples collected in Peru showed that pfhrp2-negative parasites have multiple origins, and possibly evolved independently [Sci Rep. 2013, doi: 10.1038/srep02797]. Prevalence of this character is highly variable in other parts of the world [WHO, Global Malaria Program 2016]. The differential diagnoses of an acute febrile illness in a patient coming from the north coast of Peru include malaria, dengue, typhoid fever, brucellosis and leptospirosis. When considering dengue, the absence of a rash and the duration of the fever made the diagnosis less likely. Typhoid fever and brucellosis are farther down in the list of differential diagnoses due to the patient’s clinical course. Typhoid does not typically present with thrombocytopenia, whereas brucellosis typically presents with arthralgia. Leptospirosis is usually accompanied by marked jaundice and renal failure. In Peru, there has been an average of 2,530 cases of malaria reported annually from 2015 to 2019, most of them in the Amazon region [CDC Peru]. Management approach relies on the species identified, resistance to antimalarial drugs, and severity of disease. Oral treatment with artemisinin-based combination therapy (ACT) for 3 days is the recommended first-line treatment against both P. vivax and P. falciparum. In P. vivax infection, a 14-day course of primaquine should be added to prevent recurrence. In Peru, due to a low prevalence of G6PD deficiency, patients are not typically tested prior to treatment with primaquine. If there is concern for severe malaria, intramuscular or intravenous artesunate should be used for at least 24 hours and until patients can tolerate oral therapy, then complete treatment with 3 days of ACT. [WHO 2015] WHO defines severe malaria as the presence of one or more of the following clinical indicators: deep coma suggestive of cerebral malaria, prostration or generalized weakness with inability to walk, two or more seizures within 24 hours, pulmonary edema, ARDS, shock, significant bleeding. It may also be accompanied by laboratory indicators such as severe malarial anemia (Hct: <20%, Hb: <7g/dL), renal failure (serum creatinine >3mg/dL), hypoglycemia (<40mg/dL), hyperparasitemia (>10%), acidosis and hyperbilirubinemia (serum bilirubin >3.0mg/dL). Progression to severe and fatal disease is largely but not entirely confined to P. falciparum. In the case presented, the patient was judged to be at high risk for progression to severe malaria due to hypotension at presentation and altered renal function tests, serum bilirubin, and lactate. Strictly speaking, he did not fulfill WHO criteria for severe malaria; however, he did not tolerate oral medications at the time of admission, and so was started on IV artesunate, of which he received a total of 3 doses. Afterwards, he received a full 3-day course of oral artesunate-mefloquine, followed by 7 days of oral primaquine. In Peru, most cases are caused by P. vivax or P. falciparum, with occasional reports of P. malariae. Official Peruvian data report mixed infection in 17 of 54,627 reported malaria cases for 2016 [CDC Peru]. However, studies from malaria endemic regions in Southeast Asia report nested-PCR-detected rates of mixed infection as high as 23.5%, where microscopy methods only detect 0-2.5% [Indian J Med Res 2010; 132:31-5]. Underreporting of mixed malaria infection is frequent, due to limitations in diagnostic methods. It is important to accurately diagnose mixed malaria infections because treatment may need to be altered in order to ensure clearance of parasitemia [Malar J 2011, doi: 10.1186/1475-2875-10-253]. One of the most important, although rare, complications of severe malaria is secondary bacterial infections. Increased risk of bacteremia in children with severe malaria has been previously described [Lancet 2011, doi: 10.1016/S0140-6736(11)60888-X]. Bacteria isolated in malaria patients (usually P. falciparum) include Salmonella (typhi, typhimurium, paratyphi), Staphylococcus aureus, and Escherichia coli [Am J Trop Med Hyg 2018, doi: 10.4269/ajtmh.18-0378, Open Forum Infect Dis 2016, doi: 10.1093/ofid/ofw028]. Our patient was started on IV ceftriaxone and clarithromycin because of the new consolidation on the chest X-ray, with marked clinical improvement over the next days. |