 |
Gorgas Case 2025-10 |
 |
|
The following patient was seen in the Department of Infectious and Tropical Medicine at Hospital Cayetano Heredia during the 2025 Gorgas Advanced Course.
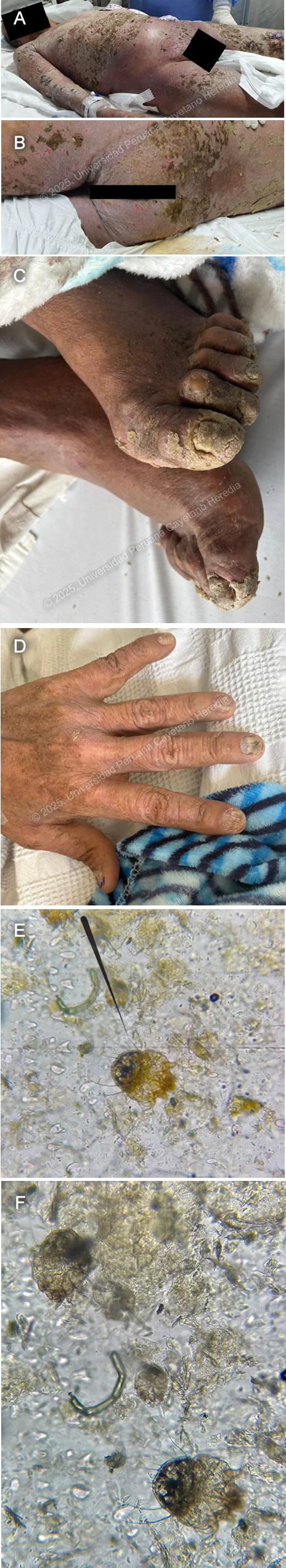 History: A 68-year-old male patient with a history of benign prostatic hyperplasia presented to the emergency department with a 7-month history of scaly skin lesions spread across the body, along with severe itching. Seven months prior to admission, the patient developed itchy, scaly lesions on both lower legs. Six months before admission, similar lesions appeared on the upper extremities and chest, accompanied by worsening itching. The skin lesions gradually spread, eventually covering the entire body surface. Initially, he was presumptively diagnosed with psoriasis at another facility and was started on oral medication with no clinical improvement. The lesions on his feet and hands became painful and limited his mobility, prompting him to seek emergency care. Epidemiology: The patient was born in Ancash, a city located at 3,052 meters above sea level in the highlands of Peru, where he lived until he was 5 years old. He currently resides in Lima. He reports traveling regularly through the jungle since 1980. He works as a cleaning staff member at churches. He reports frequent alcohol consumption and use of inhaled drugs. Physical Examination on admission: BP: 130/60, RR: 17, HR: 94, Sp02 98% on room air, T 37.6°C. The patient was not in acute distress. Thick, whitish, crusted plaques with an erythematous base, some exhibiting surface fissures and erosions, were seen on the arms, anterior chest wall, and abdomen. The palmar surfaces revealed several scaly papules and plaques involving the palmar creases. Fingernails exhibit nail dystrophy, onychorrhexis, and thickening of the nail plates. All toenails demonstrated nail plate thickening, onychodystrophy, and xanthonychia. (Images A,B,C,D). The rest of the exam was non-contributory. Laboratory: Hemoglobin 12.8g/dL; hematocrit 37%; WBC 36000/µL with bands 0%, neutrophils 70%, eosinophils 14% (absolute 1900/µL), basophils 0%, monocytes 7% and lymphocytes 9%. Platelets 406000/µL. Creatinine 0.95 mg/dL; urea 33 mg/dL; AST 24 IU/l; GGT 18 IU/L. INR was 1.14. Glucose 112 mg/dL; sodium 137 mEq/l; potassium 3.86 mEq/l, and chloride 102 mEq/l. Serology for HIV, hepatitis C, and hepatitis B were negative. The HTLV-1 chemiluminescent immunoassay was positive. Chest X Ray was non-contributory. Skin scrape microscopy is shown in Images E,F. UPCH Case Editors: Carlos Seas, Course Director / Paola Nakazaki, Associate Coordinator |
|
Discussion: The skin scrape microscopy shows structures typical of Sarcoptes scabiei. The Human T-cell Lymphotropic Virus type 1 is a retrovirus of the Retroviridae family. First identified in the early 1980s, it is known to exhibit a strong tropism for CD4+ T lymphocytes and establishes a persistent, lifelong infection in the host (1). Transmission occurs primarily through vertical routes such as breastfeeding, as well as through horizontal mechanisms including sexual contact, blood transfusion, and intravenous drug use (1,2). HTLV-1 infection is a neglected tropical disease with an estimated prevalence of 5 to 10 million carriers globally. It is endemic in certain regions, including southwestern Japan, the Caribbean, parts of South America, sub-Saharan Africa, and areas of the Middle East and Australia. Despite its clinical significance, HTLV-1 remains under limited public health surveillance and lacks effective antiviral therapies or vaccines (1,3). Although many infected individuals remain asymptomatic, the virus can cause severe diseases such as adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). HTLV-1 infection induces a chronic state of immune dysregulation, predominantly affecting cell-mediated immunity via alterations in CD4⁺ T-cell subset polarization, impaired cytotoxic T lymphocyte responses, and dysregulated cytokine networks. These immunologic perturbations predispose individuals to a distinct spectrum of infectious diseases, many of which exhibit increased severity, chronicity, or dissemination in the context of HTLV-1 seropositivity (1). The most well-established and clinically significant association is with Strongyloides stercoralis with a marked increased risk of developing the hyperinfection syndrome (see Case of the Week 03-2012), characterized by uncontrolled proliferation and dissemination of larvae, often leading to severe gastrointestinal, pulmonary, and systemic complications (4, 5). An increasingly recognized clinical association exists between Sarcoptes scabiei var. hominis hyperinfestation, commonly referred to as crusted scabies, and HTLV-1 infection, as its immune dysregulation contributes to both susceptibility and severity of infestation (6). The extreme mite proliferation, reaching millions per host, renders the condition highly contagious, with transmission possible via minimal direct contact or fomite exposure. It is characterized by extensive hyperkeratotic skin lesions, often with overlying crusts and fissures that can be distributed across the scalp, face, trunk, extremities, and periungual regions. Nail dystrophy and subungual hyperkeratosis are frequently observed, and in severe cases, the infestation may extend to mucocutaneous junctions. It can be accompanied by pruritus; however, it can be absent or attenuated due to blunted inflammatory responses in individuals with impaired cell-mediated immunity. Crusted scabies presentation can also mimic other dermatologic conditions such as psoriasis, eczema, or keratoderma, complicating clinical diagnosis (7). During hospitalization, our patient presented with fever, and developed Staphylococcus aureus (MSSA) bacteriemia. Secondary bacterial colonization, often with Staphylococcus aureus or Streptococcus pyogenes, is a common case of crusted scabies and can precipitate systemic sequelae, including bacteremia and sepsis (7). Currently, there is no curative or antiviral therapy available that can eliminate HTLV-1 infection. Management is therefore focused on treating HTLV-1–associated diseases and providing supportive care. Treatment approaches vary significantly depending on the clinical manifestation, most notably adult T-cell leukemia/lymphoma (ATLL), depending on the clinical subtype, and HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP), with limited evidence (8, 9). Prophylactic antiparasitic therapy with ivermectin is recommended in endemic areas to prevent Strongyloides hyperinfection (5). Public health strategies focus on preventing transmission, including blood donor screening, avoidance of breastfeeding by infected mothers, and safe sexual practices (1). Monitoring the proviral load, neurological progression, and oncogenic transformation is essential in long-term management. Treatment should be combined with environmental decontamination and isolation precautions to prevent the transmission of the disease. Our patient was started on ivermectin for 3 days and repeated the dose for three more days. One week later, permethrin 5% was applied to the lesions all over the body daily. MSSA bacteremia is being managed with oxacillin. References |