 |
Gorgas Case 2025-4 |
 |
|
The following patient was seen in the inpatient ward of Cayetano Heredia Hospital in Lima by the 2025 Gorgas Course participants.
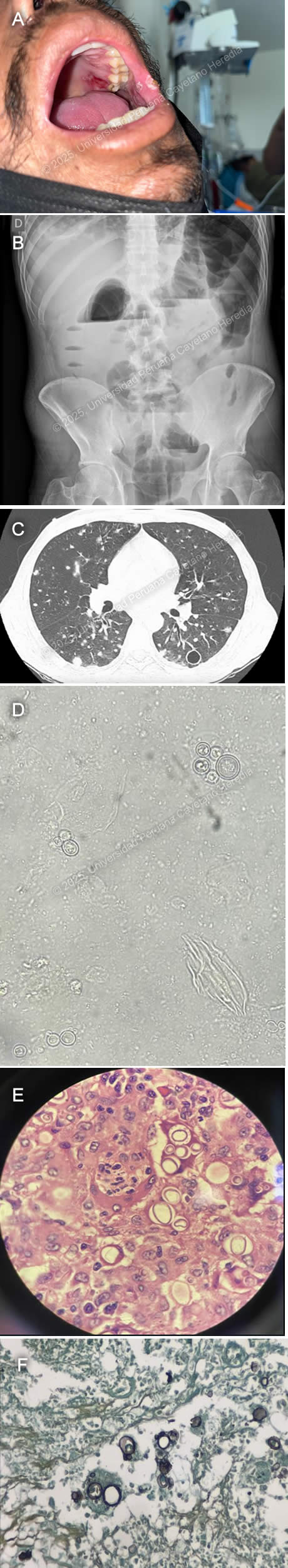 History: A 43-year-old male patient with no significant past medical history presented to the emergency department of Cayetano Heredia Hospital (HCH) with 2.5 months of diarrhea, vomiting, and abdominal pain. Two months and fifteen days before admission, he presented with watery diarrhea without mucus or blood, 3-4 times every other day, associated with postprandial abdominal pain. Two months before admission, he attended a Primary Care Center, where he tested positive for HIV and was promptly started on ART (tenofovir, lamivudine, dolutegravir). His symptoms remained stable with marginal improvement, and one month before admission, he developed marked abdominal distention associated with postprandial vomiting. This was aggravated by the appearance of lower extremity edema. On the day of admission, he presented with worsening abdominal pain. An ambulatory colonoscopy revealed multiple ulcers in the descending, sigmoid, and ascending colon and cecum, including fistulizing ulcers in the descending and sigmoid colon. Of note, he had a 15 kg involuntary weight loss in the last 6 months and 2.5 months of asthenia. He denied headaches, visual symptoms, fever, dyspnea, cough, or chest pain. Epidemiology: The patient was born in Cerro de Pasco, a city located in the central highlands of Peru, but currently lives in northern Lima, where he moved 25 years ago after working for one year as a coffee farmer in the jungle of Chanchamayo. He denies any known TB contacts and has no pets. He has had five male sexual partners with inconsistent condom use and was previously treated for genital herpes one year ago. Physical Examination on admission: BP: 110/70 mmHg, RR: 18, HR: 115, Temp: 36.1°C, SpO₂: 96% on room air. He appeared malnourished but was ventilating spontaneously and in no acute distress. An approximately 2 × 2 cm ulcer in the right upper hard palate and 2-4 smaller ulcers in the left internal cheek were found (Image A). Pitting edema up to his knees was noted, and had multiple palpable 0.5 × 0.5 cm mobile, non-tender cervical lymph nodes. He had normal breath sounds with diffuse wheezing but no rales or crackles. His abdomen was markedly distended, with increased bowel sounds and shifting dullness. The rest of the exam was normal. Laboratory: Hemoglobin 9.9 g/dL (MCV 79.6 fL, MCHC 35.6 g/dL), WBC 8,300/uL (0% bands, 77.2% neutrophils, 0.1% eosinophils, 0.1% basophils, 8.6% monocytes, and 14% lymphocytes), platelets 380,000/uL. Urea 31 mg/dL, creatinine 0.64 mg/dL. AST 31 U/L, ALT 28 U/L, alkaline phosphatase 108 U/L, total bilirubin 0.5 g/dL, LDH 211 U/L. INR 1.23. A repeat HIV test was positive, and RPR, hepatitis B, and C serologies were negative. An initial HTLV-1 ELISA test at a reference lab was positive, but an in-house repeat ELISA was negative, confirmatory Western Blot is pending. A thorough TB workup was negative, including sputum AFB and GeneXpert ultra, urinary TB LAM, and tuberculin skin test. The serum cryptococcal lateral flow assay was negative, as were serial stool O&P’s. Lastly, a urinary Histoplasma sp. antigen was found to be positive. Imaging: An abdominal ultrasound revealed an estimated 1-1.5 L free intraperitoneal fluid collection and hepatomegaly. An abdominal X-ray showed dilated small and large bowel loops and multiple air-fluid levels (Image B). An abdominal CT scan showed dilated intestinal loops associated with bowel wall edema. A chest CT showed multiple bilateral nodules associated with areas of ground-glass opacities and multiple thin-walled cavitary lesions (Image C). A KOH stain from a BAL sample was performed (Image D). Results from biopsies obtained from the colonoscopy are shown (Image E, H&E stain; Image F, Grocott stain). UPCH Case Editors: Carlos Seas, Course Director / Mario Suito, Associate Coordinator |
|
Discussion: Both the direct BAL smear and the large intestine biopsies revealed variable-sized yeasts with narrow-based budding and birefringent walls, findings compatible with Paracoccidioides sp. infection. PCM is an endemic mycosis of Latin America’s rainforests caused by the thermally dimorphic fungus Paracoccidioides brasiliensis and, less commonly, by Paracoccidioides lutzii (1). Brazil reports 80% of all cases, with the remainder being from Argentina, Bolivia, Colombia, Ecuador, Venezuela, Mexico, and Peru (2). Transmission occurs through the respiratory route, with an estimated 10 million people infected in Latin America, most of them being over 30 years old, with no racial predominance but with a male-to-female ratio of around 14:1 (3). The most associated risk factor is working as a farmer in coffee or tobacco crops (4). PCM can be clinically divided into asymptomatic infection, active disease, and sequelae or residual form. Active PCM disease can be further classified into an acute/subacute or juvenile form, a chronic progressive or adult form that can be unifocal or multifocal, or a mixed form with characteristics of both the juvenile and chronic progressive forms. The juvenile form develops in days to weeks and commonly presents with fever and reticuloendothelial system involvement (generalized lymphadenopathy, liver and spleen enlargement). At-risk populations are those less than 30 years old and immunosuppressed patients, such as those with advanced HIV (5). The chronic progressive form develops over months to years and commonly presents with bilateral, apical-sparing pulmonary involvement, localized lymphadenopathy, and oral mucosal lesions. Pulmonary symptoms are usually mild, with hemoptysis being rare. Radiological findings can include alveolar, interstitial, and/or nodular patterns. Cavitary lesions and pleural effusions are less common. Pulmonary fibrosis is the eventual common endpoint for severe and/or untreated PCM pulmonary disease (6). Mucosal lesions are more common in the chronic form and usually involve the oral mucosa. Lesions tend to be painful, destructive, and ulcerative, accompanied by gingival infiltration causing tooth loss. Skin lesions are more common in the juvenile form, are pleomorphic, and frequently affect the face. Intestinal involvement can affect any segment of the GI tract but most commonly affects the ascending colon, cecum, and ileum, followed by the small intestine. Symptoms include abdominal pain, weight loss, and chronic diarrhea (7). Patients with advanced HIV and CD4 counts less than 200 are at risk for the reactivation of latent foci. These patients tend to present with disseminated, multi-organ involvement, exhibiting characteristics of both the chronic multifocal and juvenile forms (8). Of note, HTLV-1 coinfected patients have also been described with a predominance of juvenile and/or mixed forms and intestinal involvement (9). The fastest, most cost-effective, and clinically applicable diagnostic method is the direct examination of round yeast cells with peripheral budding in KOH-prepared samples from affected tissues, such as oral or skin lesions, sputum, lymph node aspirates, and CSF (10). The same yeast cells can be seen in histopathological samples, which can be enhanced by silver stains, PAS, and/or immunofluorescence techniques. Culture is the second standard diagnostic method but requires cool temperatures of 18-24°C and takes up to 20-30 days to grow. Serological tests remain an area of ongoing research, being utilized in endemic countries like Brazil but unavailable in most settings. Antibody detection using the double immunodiffusion method (DID) with the 43 kDa antigen is the most used method but remains positive years after exposure, limiting its use in detecting active disease (11). For mild to moderate cases of PCM, the preferred treatment option is itraconazole, with a variable duration of treatment from 9 to 18 months. Cotrimoxazole can also be used for 18 to 24 months. In cases of severe and disseminated presentation, the preferred therapeutic choice is 2 to 4 weeks of Amphotericin B until clinical stabilization, followed by a switch to oral maintenance therapy (11). Our patient was started on amphotericin B 1.0 mg/kg. References |