
Click image to enlarge
Graphic: Jody PotterThe word xenotransplantation — or the concept of it — is new to most people and many have lingering questions for its future. Xenotransplantation is the transplantation of organs or tissues from an animal source into a human recipient. Researchers and surgeons have been working towards viable xenotransplant options for decades, in the hopes of being able to save more live and improve the quality of life of their patients.
The University of Alabama at Birmingham recently announced the first peer-reviewed research outlining the successful transplant of genetically modified, clinical-grade pig kidneys into a brain-dead human. The study recipient had two genetically modified pig kidneys transplanted in his abdomen after his native kidneys were removed. The organs were procured from a genetically modified pig at a pathogen-free facility.
This process demonstrates the long-term viability of the procedure and how such a transplant might work in the real world. The transplanted kidneys filtered blood, produced urine and, importantly, were not immediately rejected. The kidneys remained viable until the study was ended, 77 hours after transplant.
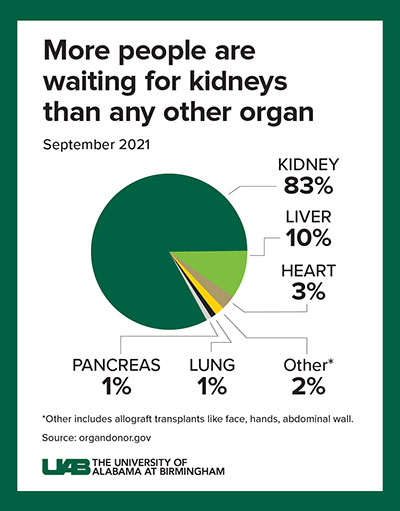
“While these positive results demonstrate how xenotransplantation could address the worldwide organ shortage crisis — more than 800,000 Americans who live with kidney failure and more than 90,000 who are waiting for a kidney transplant — there is much curiosity surrounding what this procedure looks like and what it means for the future of science in this field,” said Jayme Locke, M.D., director of the Comprehensive Transplant Institute in UAB’s Department of Surgery and lead surgeon for the study. “We have a long way to go before xenotransplantation is available to the masses, but we will push forward into the phase of making this breakthrough treatment a reality.”
As the science of xenotransplantation moves forward, many questions have been raised of what the future holds.
What has UAB achieved?
UAB achieved the first inside-the-body transplant of genetically modified pig kidneys. The study was conducted with the permission of an Alabama family and has provided important safety information. Importantly, the study simulates how this type of transplant would be performed in a living human.
“We were able to model every step of the process was exactly as it would be done if it was a phase I clinical trial, which is why we feel confident in its success,” Locke said. “Without question this study has established brain death as a pre-clinical human model to study the human condition, extending far beyond xenotransplantation. There are many diseases we need to understand and work through new techniques and devices. Animal models are not always sufficient. Being able to test this in a brain-dead human before it goes into a living person is remarkable and allows us to capture key safety end points.”
Click image to enlarge
Graphic: Jody Potter
Why is this significant? How does this change the future of transplant?
There is an acute shortage of organs in our country with the most common organ being the kidney. Kidney disease kills more people each year than breast or prostate cancer, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Although transplantation is the gold standard treatment for end-stage kidney disease, fewer than 25,000 kidney transplants are performed each year in the United States and 240 Americans on dialysis die every day. Many of these deaths could be prevented if an unlimited supply of kidneys were available for transplant.
The wait for a deceased donor kidney can be as long as five years, and in many states, it is closer to 10 years. Almost 5,000 people per year die waiting on a kidney transplant.
“Kidney transplant is a cure for end stage kidney disease, dialysis is not,” Locke said. “We have a cure, we understand it, but we have a huge gap between supply and demand. This is a refractory, unmitigated crisis and radical solutions are needed. We believe that xenotransplantation affords the opportunity to identify a novel organ source and overcome the organ shortage and provide a kidney for every person in need.”
What makes what this study accomplishment unique?
This study is the:
- First peer-reviewed, published study of a genetically modified pig kidney transplanted into the body of a brain-dead human recipient
- First such study on a pig-to-human kidney transplant using genetically modified kidneys with 10 key gene edits that may make the kidneys suitable for direct clinical-grade therapeutic use in humans
- First validation of a UAB-developed test for compatibility before xenotransplant
- First peer-reviewed, published study to establish brain death as a viable preclinical human model
Notably, the study was designed and conducted to meet standards directly comparable to those that would apply to a Phase I clinical trial. It mirrored — as much as possible — every step of a conventional transplant between humans. Importantly, this study included removing the human brain-dead recipient’s native kidneys before replacing them with genetically modified pig kidneys.
When would UAB be able to take this into a living human clinical trial?
UAB physicians are already working on the next steps to begin a clinical trial or compassionate or emergency use of the pig kidneys in living humans. Two major approvals will be required.
First, the U.S. Food and Drug Administration must approve an investigator-initiated Investigational New Drug Application to administer the biological product — genetically modified pig kidneys — to humans. The U.S. Food and Drug Administration protects public health by ensuring the safety and efficacy of drugs and biological products.
Secondly, UAB’s Institutional Review Board for Human Use, established by federal regulations to protect human subjects in research, must review and approve the proposed clinical trial before the start of a Phase I trial to test that this transplant is safe in living humans.
“We anticipate getting additional guidance from the FDA about what they think is appropriate soon,” Locke said. “It is quite possible that they may want us to do additional studies in the human brain-dead model. We plan to move this model forward, and look to perform additional transplants in this model to continue to collect and compile data to make the case that we are ready to do this in living people.”
What are the next steps before these 10-gene-edit pig kidneys can be used for transplant?
UAB and other academic collaborators continue to generate data, including thorough pre-clinical work as we progress the science behind xenotransplantation.
“We are continuing to work with the FDA on a clinical and regulatory path forward,” Locke added. “The present study that was published answered critical safety questions that we needed to have answers to in order to perform the transplant into living persons.”
How much closer does this put us to being able to safely perform xenotransplantation in living humans, and how soon until we get there?
An exact timeframe will depend on approvals, successes in future studies and progression within clinical trials. “We are confident that the success of this transplant, our published findings and successful xenotransplants at other institutions are getting us as close as possible to this being a viable option for patients in need,” Locke added.
Why pigs? What similarities exist between pigs and humans?
Pig kidneys are very similar to human kidneys in their makeup and are about the same size.
“When you think about trying to ramp up xenotransplant in relatively short order and keep pace with the demand, the pig offers a great opportunity for that,” said Locke. “Of course, pig organs are quite naturally foreign to the human immune system. For that reason, it is important that these 10 gene edits occur so that the pig kidney becomes a bit more human. This allows the human immune system along with standard immunosuppression that we use in human-to-human transplantation to tolerate the pig kidney and sustain in the person via transplant.”
Will xenotransplant replace allotransplantation (human-to-human transplants)?
No, xenotransplant is meant to be complementary to human-to-human transplantation.
Locke adds that people who are compatible for a xenograft from a pig, paired with living and deceased human-to-human allotransplantation, could potentially wipe out the kidney transplant waiting list.
“Xenotransplantation is simply filling the gap that allotransplantation cannot quite fill, but it needs to be done in concert,” Locke said.
How long is it anticipated to take before the necessary approvals are granted?
The timeline will depend on the interactions with the FDA and UAB’s IRB on a clinical path forward.
“We feel that we should pursue the fastest and safest path forward to bring these groundbreaking technologies to patients in need,” Locke said. “We are hopeful that we can begin the phase I clinical trial in humans soon.”
To learn how to become a registered organ donor, visit Legacy of Hope’s official website.
Are there any potential dangers of using an animal organ in a human? Is this process sanitary?
Yes, this is a sanitary process as the pigs are raised in a pathogen-free facility. Additionally, the modification of the pig’s genes makes their organs less likely to be rejected by a human recipient. However, the risk of infections like porcine retrovirus, a pig virus that may be transmitted to humans, is considered very low risk.
“Revivicor pig lines are bred to be free of PERV C, so we see little chance of a PERV A x PERV C recombination event infecting human patients,” Locke said. “The FDA, going back 10 years, has embraced this as sufficient.”