Does my study require CBR Review?
Submission for full fiscal approval process review is required for any clinical trial/research project that includes clinical billable activities that are provided and billed by the UAB Health System.
-
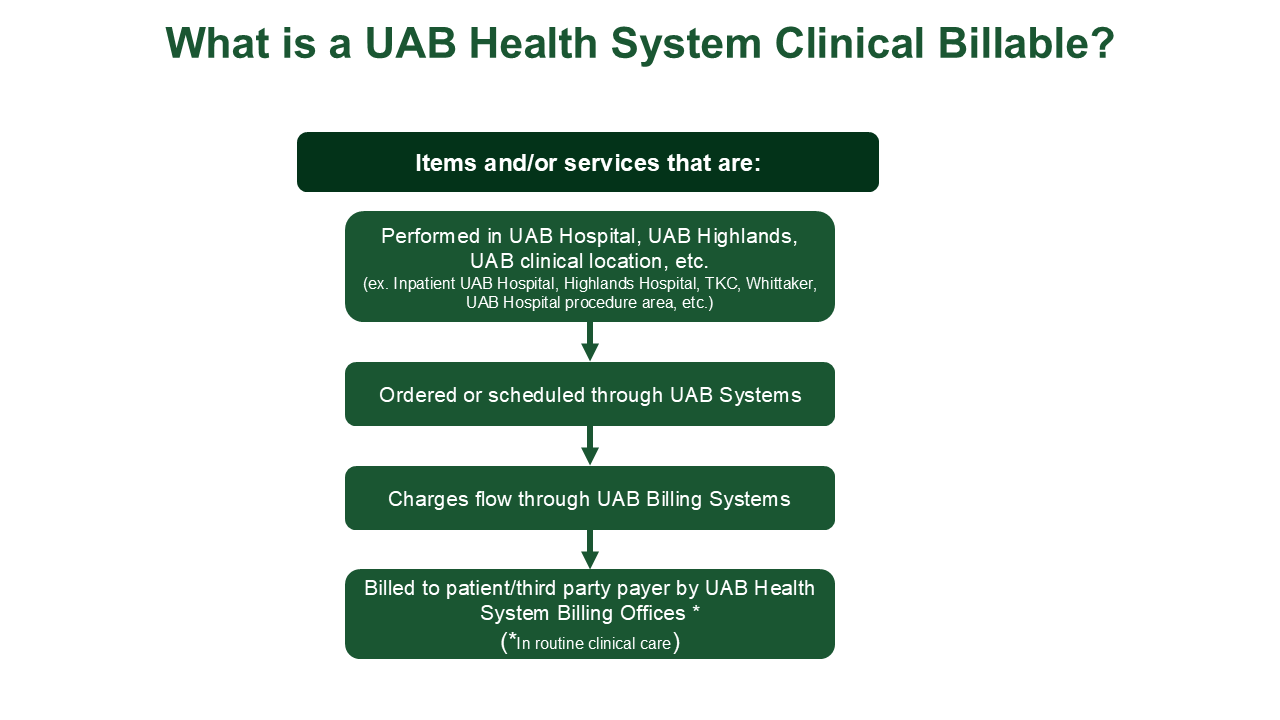
What is a UAB Health System (UABHS) Clinical Billable?
Any clinical service performed during the conduct of a research study (on the protocol schedule of events or listed as a procedure on your IRB Human Subjects Protocol) that is billed through the UAB Clinical Billing Offices (MSO/HSF and/or PFS).
NOTE: UAB Health System Billables may be billed to the research study and/or to insurance. If any UABHS Clinical Billable Services are billed to insurance, the trial must be reviewed by CBR.
Examples of UABHS Clinical Billable Services Procedure Performed By Billed By X-Rays, MRIs, CT Scans or other Radiological Procedures TKC Staff, Hospital staff, Whittaker Staff, Highlands Staff, etc – UAB Radiology UAB Health System Billing Offices ECG TKC Staff, Hospital Staff, Whitaker Staff, etc UAB Health System Billing Offices Echo TKC Staff, Hospital Staff, Whitaker Staff, etc – UAB Echocardiographics UAB Health System Billing Offices Pulmonary Function Tests TKC Staff, Hospital Staff, Whitaker Staff, etc UAB Health System Billing Offices Local Labs UAB Hospital Labs
(*NOTE: If local labs are performed in CRU, they may be resulted by UAB Hospital Labs – check with CRU or CBR if questions)UAB Health System Billing Offices Shipping and Processing to a Central Lab UAB Hospital Labs UAB Health System Billing Offices
Examples of Non-UABHS Clinical Billable Services Procedure Performed By Billed Clinical Procedures Performed in a Research Lab or Research Unit that does not bill through the UAB Health System Research Staff (Research Nurse, PI, etc) N/A – not billable through the UAB Health System X-Rays, MRIs, CT Scans or other Radiological Procedures performed outside the standard for this scan (scan itself or just the data transfer fees, etc)
(*NOTE: Questions - Rad Research:This email address is being protected from spambots. You need JavaScript enabled to view it. )Research Radiology By Research Radiology directly to the Study Team ECG Study Team N/A Central Labs
(*NOTE: If local labs are performed in CRU, they may be resulted by UAB Hospital Labs – check with CRU or CBR if questions)Study Team N/A Shipping and Processing to a Central Lab Study Team N/A -
What is Coverage Analysis?
Coverage Analysis is the review of the proposed UAB Health System Billable Items/Services to ensure appropriate billing to insurance or the study, per the CMS NCD 310.1 (Routine Costs in Qualifying Clinical Trials) which states,
“Effective for items and services furnished on or after July 9, 2007, Medicare covers the routine costs of qualifying clinical trials, as well as reasonable and necessary items and services used to diagnose and treat complications arising from participation in all clinical trials. All other Medicare rules apply.”
Coverage Analysis Includes:- Determining and documenting whether or not the trial is Qualifying per Medicare rules (Study Type)
- Documenting routine care for UAB Health System items/services planned to be billed to insurance and confirm that the item/services meets indications and limitations for Medicare coverage
- Confirmation of CMS Approval for Category A, Category B Device trials and CED trials
- Review of the informed consent document to ensure there is no problematic language (the study team/sponsor may not pick up the cost of an item/service if insurance does not cover/denies; if promised free in the consent, insurance may not be charged for item/service; costs to the participant need to be clearly communicated in the consent form, etc.)
- Communication to the study team, the Human Research Protection Program (HRPP) and the Office of Sponsored Programs (OSP) any billing limitations and/or problematic language (the study team/sponsor may not pick up the cost of an item/service if insurance does not cover/denies; if promised free in the consent, insurance may not be charged for item/service; costs to the participant need to be clearly communicated in the consent form, etc.)
- Facilitate coding and pricing for UAB Health System Clinical Billable Services between the Clinical Billing Offices (CBO), Revenue Integrity, and the Study Team
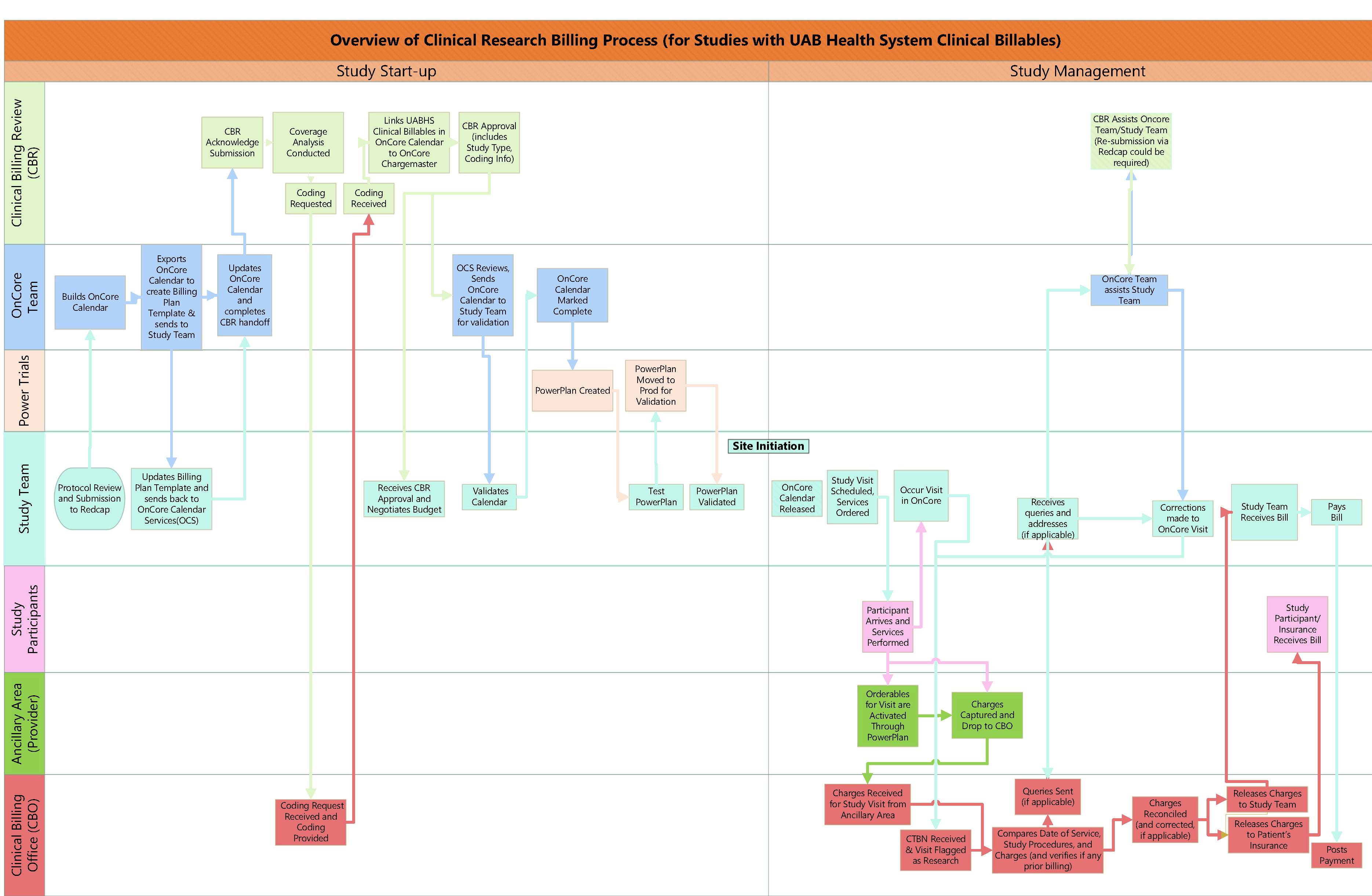
- What does the UAB Billing Process look like?
How do I submit for CBR Review?
If a study has UAB Health System clinical billable services, CBR review, an OnCore Calendar, and OnCore study management is required. You will submit to the same link for both CBR review and for an OnCore Calendar build.
-
New Studies
Full Fiscal Approval Process Review Submission
Submission for full fiscal approval process review is required for any clinical trial/research project that includes clinical billable activities that are provided and billed by the UAB Health System.
To submit a request for review for studies involving UAB Health System Clinical Billable Services, OnCore Calendar Requests, and/or UAB Health System Clinical Billable Services, please go to this link. Complete the information, as applicable in the form.
Please note: you will need all required information on hand before submission. Please see the checklist at this link (BlazerID email/password for login) in order to determine needed information for review before you begin your submission. If available at the time of submission, please submit the copy of the Clinical Research Unit (CRU) award letter (please submit when available).
Link to workflow (blazerID/password required). -
Device Trials
Device Trial Submission
Submission for Device Trial is required for any clinical trial/research project that involves the use of any type of device (Category A, Category B, Non-Significant Risk, 510K Summary, Wearable Device, PMA, etc.) for which clinical billable activities are provided and billed by the UAB Health System.
Hospital LOA Requirement: The study information will be submitted by CBR on your behalf to the Hospital Administration for review to determine if a Letter of Agreement (LOA) will be required. Note: for questions regarding Hospital Letter of Agreement, please contact Leigh Wright (This email address is being protected from spambots. You need JavaScript enabled to view it. ) or Christy Fancher (This email address is being protected from spambots. You need JavaScript enabled to view it. )
Device Trials: Fill out the appropriate information in the online Redcap application to submit a request for review for studies involving UAB Health System Clinical Billable Services, OnCore Calendar Requests, and/or UAB Health System Clinical Billable Services.
Please note: you will need all required information on hand before submission. Please see the checklist at this link (BlazerID email/password for login) in order to determine needed information for review before you begin your submission. -
Amendments
Amendment Review
Amendment Review: If you have had a study managed by any of the following groups in the past, and now there is a *change you will complete the electronic forms in the REDCap application, and click "Revision":- Clinical Billing Review/FAP
- OnCore / Oncore Calendar Services (OCS)
- CCTS (CRU, SPAN, Bionutrition, CRSP, etc)
Link to workflow (blazerID/password required).
NOTE: If research activities are being provided by an Ancillary Research Area that bills for their services internally - department to department (outside of the UAB Health System), please contact the ancillary research area directly for research pricing.
How do I get pricing and coding for my study?
CBR does not obtain fees for research services provided by areas that do not bill through the UAB Health System. The areas who do not bill through the UAB Health System are referred to as ancillary research areas. Departments performing a study whose activities use an ancillary research area should contact the ancillary research area directly regarding their research fees for non-clinical billable services.
-
Only need pricing and coding for UABHS clinical billable Services?
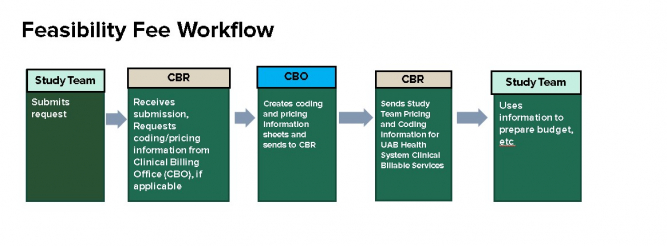
Feasibility Fee Request Submission
Departments may choose to submit a Feasibility Fee Request to CBR in order to:- obtain preliminary research fees for protocol-required clinical billable activities to perform cost feasibility evaluations; OR
- obtain research fees for protocol-driven clinical billable activities when developing a proposed budget for grant/industry study proposals.
Feasibility Fee Request: To submit a Feasibility Fee Request, fill out the appropriate information in the online Redcap application. Please make sure to list the UAB Health System Clinical Billable Services for which you need pricing, and the location of the service.
The request will be sent to the Clinical Billing Offices (CBO), and pricing/coding information will be returned to you as soon as it is received.
Notes: Should you submit a Feasibility Fee Request, and the study/project moves forward for activation at UAB, then a Full FAP Review submission is required at that time.
If research activities are being provided by an Ancillary Research Area that bills for their services internally - department to department (outside of the UAB Health System), please contact the ancillary research area directly for research pricing.
Last updated: December 10, 2025