Tissue collection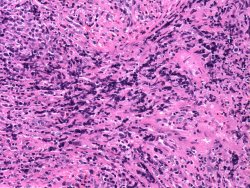
Collect tissues using sharp scissors or scalpel blades and mouse-tooth forceps. Handle tissues gently. Grasp and cut samples at the edges, so the areas of interest aren't damaged. At right is a section of mouse spleen damaged by rough handling. Many of the cells are distorted beyond recognition.
Fixation
There are many fixatives and many considerations in their use. For routine paraffin processing and most stains, neutral buffered 10% formalin is a good choice. In general, mouse tissues should be fixed in formalin for 24 to 48 hours, but can be left much longer. Our standard fixative for rodent tissues is 75% ethanol-10% formalin. The ethanol speeds fixation and stiffens tissues such as lung for easier trimming. Bouin's is good for mouse embryos and fetuses, but is not as forgiving, and the tissues must be adequately rinsed after fixing to remove the picric acid. Fixatives can be purchased from vendors of scientific supplies. Contact us if you're not sure what fixation protocol to use
Use plenty of fixative!
In general, the ratio of fixative to tissue (vol:vol) should be 15:1 to 20:1. Dense (skin, bone) and bloody (liver, spleen) tissues are hardest to fix. If the amount of fixative is inadequate, or the sample is too large or too thick, the interior of the specimens won't be properly fixed. Inadequate fixation is one of the most common problems we encounter.
Fresh tissues tend to stick together and to the bottom of the container, which inhibits fixation. Thus, it's a good habit to swirl the containers a time or two to separate the tissues from each other and from the container bottom. If there is a 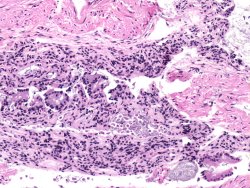 lot of blood in the fixative after tissue collection, replace it with fresh fixative.
lot of blood in the fixative after tissue collection, replace it with fresh fixative.
Tissues must be placed in fixative promptly to prevent autolysis. Refrigerating the fixative and specimen containers slows autolysis. Sometimes additional procedures are needed. For example, lungs might need to be infused with fixative to approximate the normal state of inflation, and intestines may need to be injected with fixative or opened for optimal fixation. At right is a section of mouse large intestine in which the mucosal morphology is lost due to autolysis.