Center News
UAB Comprehensive Diabetes Center 2022 Research Roundup

The UAB Comprehensive Diabetes Center is comprised of faculty members conducting basic, translational, and clinical research related to diabetes and its complications.
Current areas of research include autoimmune type 1 diabetes, type 2 diabetes, diabetes complications, beta cell biology, oxidative stress, signaling and metabolism as well as diabetes risk factors, epidemiology, intervention studies, outcomes research, and drug discovery.
With funding from national institutes, foundations, and donors, we perform cutting-edge diabetes research, provide comprehensive education and training, and uncover breakthroughs in treatment for those affected by diabetes in our community and beyond. Below is a list of examples of recent key peer-reviewed publications from the UCDC Young Diabetes Principle Investigator group and UCDC director and associate directors.
- LDB1-mediated transcriptional complexes are sensitive to islet stress.
Liu Y, Kepple JD, Shalev A, S Hunter C.Islets. 2022 Dec 31;14(1):58-68. doi: 10.1080/19382014.2021.2016028.PMID: 34968409
- Heterogeneity of Diabetes: β-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research's Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health's National Institute of Diabetes and Digestive and Kidney Diseases.
Cefalu WT, Andersen DK, Arreaza-Rubín G, Pin CL, Sato S, Verchere CB, Woo M, Rosenblum ND; Symposium planning committee, moderators, and speakers:, Rosenblum N, Cefalu W, Andersen DK, Arreaza-Rubín G, Dhara C, James SP, Makarchuk MJ, Pin CL, Sato S, Verchere B, Woo M, Powers A, Estall J, Hoesli C, Millman J, Linnemann A, Johnson J, Pin CL, Hawkins M, Woo M, Gloyn A, Cefalu W, Rosenblum N, Huising MO, Benninger RKP, Almaça J, Hull-Meichle RL, MacDonald P, Lynn F, Melero-Martin J, Yoshihara E, Stabler C, Sander M, Evans-Molina C, Engin F, Thompson P, Shalev A, Redondo MJ, Nadeau K, Bellin M, Udler MS, Dennis J, Dash S, Zhou W, Snyder M, Booth G, Butte A, Florez J.Diabetes Care. 2022 Jan 1;45(1):3-22. doi: 10.2337/dci21-0051.
- Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial.
Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT; STEP 8 Investigators.JAMA. 2022 Jan 11;327(2):138-150. doi: 10.1001/jama.2021.23619.PMID: 35015037
- Mother-child cardiometabolic health 4-10 years after pregnancy complicated by obesity with and without gestational diabetes.
Martin SL, Zhang L, Callahan ML, Bahorski J, Lewis CE, Hidalgo BA, Durant N, Harper LM, Battarbee AN, Habegger K, Moore BA, Everett A, Aslibekyan S, Sertie R, Yi N, Garvey WT, Chandler-Laney P.Obes Sci Pract. 2022 Feb 16;8(5):627-640. doi: 10.1002/osp4.599. eCollection 2022 Oct.PMID: 36238222
- Islet transplantation into brown adipose tissue can delay immune rejection.
Kepple JD, Barra JM, Young ME, Hunter CS, Tse HM.JCI Insight. 2022 Feb 22;7(4):e152800. doi: 10.1172/jci.insight.152800.PMID: 35015736
- Is Obesity or Adiposity-Based Chronic Disease Curable: The Set Point Theory, the Environment, and Second-Generation Medications.
Garvey WT.Endocr Pract. 2022 Feb;28(2):214-222. doi: 10.1016/j.eprac.2021.11.082. Epub 2021 Nov 22.PMID: 34823000
- Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes.
Xu G, Grimes TD, Grayson TB, Chen J, Thielen LA, Tse HM, Li P, Kanke M, Lin TT, Schepmoes AA, Swensen AC, Petyuk VA, Ovalle F, Sethupathy P, Qian WJ, Shalev A.Nat Commun. 2022 Mar 3;13(1):1159. doi: 10.1038/s41467-022-28826-3.PMID: 35241690
- New Horizons. A New Paradigm for Treating to Target with Second-Generation Obesity Medications.
Garvey WT.J Clin Endocrinol Metab. 2022 Mar 24;107(4):e1339-e1347. doi: 10.1210/clinem/dgab848.PMID: 34865050
- Subcutaneous Administration of a Nitric Oxide-Releasing Nanomatrix Gel Ameliorates Obesity and Insulin Resistance in High-Fat Diet-Induced Obese Mice.
Ren G, Hwang PTJ, Millican R, Shin J, Brott BC, van Groen T, Powell CM, Bhatnagar S, Young ME, Jun HW, Kim JA.ACS Appl Mater Interfaces. 2022 May 4;14(17):19104-19115. doi: 10.1021/acsami.1c24113. Epub 2022 Apr 25.PMID: 35467831
- Deletion of Gdf15 Reduces ER Stress-induced Beta-cell Apoptosis and Diabetes.
Xu G, Chen J, Jo S, Grayson TB, Ramanadham S, Koizumi A, Germain-Lee EL, Lee SJ, Shalev A. 2022 May 1;163(5):bqac030. doi: 10.1210/endocr/bqac030.PMID: 35290443
- FGF21-FGFR4 signaling in cardiac myocytes promotes concentric cardiac hypertrophy in mouse models of diabetes.
Yanucil C, Kentrup D, Li X, Grabner A, Schramm K, Martinez EC, Li J, Campos I, Czaya B, Heitman K, Westbrook D, Wende AR, Sloan A, Roche JM, Fornoni A, Kapiloff MS, Faul C.Sci Rep. 2022 May 5;12(1):7326. doi: 10.1038/s41598-022-11033-x.PMID: 35513431
- Associations between cardiometabolic disease severity, social determinants of health (SDoH), and poor COVID-19 outcomes.
Howell CR, Zhang L, Yi N, Mehta T, Cherrington AL, Garvey WT.Obesity (Silver Spring). 2022 Jul;30(7):1483-1494. doi: 10.1002/oby.23440. Epub 2022 May 25.PMID: 35352489
- American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD).
Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z.Endocr Pract. 2022 May;28(5):528-562. doi: 10.1016/j.eprac.2022.03.010.PMID: 35569886
- Changes in Type 2 Diabetes Trends in Children and Adolescents During the COVID-19 Pandemic.
Schmitt JA, Ashraf AP, Becker DJ, Sen B.J Clin Endocrinol Metab. 2022 Jun 16;107(7):e2777-e2782. doi: 10.1210/clinem/dgac209.PMID: 35377436
- Diagnostic Test Accuracy of Urine C-peptide Creatinine Ratio for the Correct Identification of the Type of Diabetes: A Systematic Review.
Pappachan JM, Sunil B, Fernandez CJ, Lahart IM, Ashraf AP.touchREV Endocrinol. 2022 Jun;18(1):2-9. doi: 10.17925/EE.2022.18.1.2. Epub 2022 May 23.PMID: 35949364
- Guidelines on models of diabetic heart disease.
Heather LC, Hafstad AD, Halade GV, Harmancey R, Mellor KM, Mishra PK, Mulvihill EE, Nabben M, Nakamura M, Rider OJ, Ruiz M, Wende AR, Ussher JR.Am J Physiol Heart Circ Physiol. 2022 Jul 1;323(1):H176-H200. doi: 10.1152/ajpheart.00058.2022. Epub 2022 Jun 3.PMID: 35657616
- The Ldb1 transcriptional co-regulator is required for establishment and maintenance of the pancreatic endocrine lineage.
Toren E, Liu Y, Bethea M, Wade A, Hunter CS.FASEB J. 2022 Aug;36(8):e22460. doi: 10.1096/fj.202200410R.PMID: 35881062
- Examining the evidence for weight management in individuals with type 2 diabetes.
Garvey WT, Umpierrez GE, Dunn JP, Kwan AYM, Varnado OJ, Konig M, Levine JA.Diabetes Obes Metab. 2022 Aug;24(8):1411-1422. doi: 10.1111/dom.14764. PMID: 35545861
- Cathepsin D Drives the Formation of Hybrid Insulin Peptides Relevant to the Pathogenesis of Type 1 Diabetes.
Crawford SA, Wiles TA, Wenzlau JM, Powell RL, Barbour G, Dang M, Groegler J, Barra JM, Burnette KS, Hohenstein AC, Baker RL, Tse HM, Haskins K, Delong T.Diabetes. 2022 Aug 30:db220303. doi: 10.2337/db22-0303.PMID: 36041196
- Extracellular vesicles in β cell biology: Role of lipids in vesicle biogenesis, cargo, and intercellular signaling.
Aguirre RS, Kulkarni A, Becker MW, Lei X, Sarkar S, Ramanadham S, Phelps EA, Nakayasu ES, Sims EK, Mirmira RG.Mol Metab. 2022 Sep;63:101545. doi: 10.1016/j.molmet.2022.101545. Epub 2022 Jul 8.PMID: 35817393
- Carbonyl Posttranslational Modification Associated With Early-Onset Type 1 Diabetes Autoimmunity.
Yang ML, Connolly SE, Gee RJ, Lam TT, Kanyo J, Peng J, Guyer P, Syed F, Tse HM, Clarke SG, Clarke CF, James EA, Speake C, Evans-Molina C, Arvan P, Herold KC, Wen L, Mamula MJ.Diabetes. 2022 Sep 1;71(9):1979-1993. doi: 10.2337/db21-0989.PMID: 35730902
- Cross Talk Between Insulin and Glucagon Receptor Signaling in the Hepatocyte.
Habegger KM.Diabetes. 2022 Sep 1;71(9):1842-1851. doi: 10.2337/dbi22-0002.PMID: 35657690
- Pharmacologic Weight Management in the Era of Adolescent Obesity.
Raman V, Gupta A, Ashraf AP, Breidbart E, Gourgari E, Kamboj M, Kohn B, Krishnan S, Lahoti A, Matlock K, Mehta S, Mistry S, Miller R, Page L, Reynolds D, Han JC.J Clin Endocrinol Metab. 2022 Sep 28;107(10):2716-2728. doi: 10.1210/clinem/dgac418.PMID: 35932277
- Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses.
Kosiborod MN, Bhatta M, Davies M, Deanfield JE, Garvey WT, Khalid U, Kushner R, Rubino DM, Zeuthen N, Verma S.Diabetes Obes Metab. 2022 Oct 6. doi: 10.1111/dom.14890. Online ahead of print.PMID: 36200477
- Hepatic mTORC2 Signaling Facilitates Acute Glucagon Receptor Enhancement of Insulin-Stimulated Glucose Homeostasis in Mice.
Kim T, Nason S, Antipenko J, Finan B, Shalev A, DiMarchi R, Habegger KM.Diabetes. 2022 Oct 1;71(10):2123-2135. doi: 10.2337/db21-1018.PMID: 35877180
- Alpha Cell Thioredoxin-interacting Protein Deletion Improves Diabetes-associated Hyperglycemia and Hyperglucagonemia.
Lu B, Chen J, Xu G, Grayson TB, Jing G, Jo S, Shalev A.Endocrinology. 2022 Oct 11;163(11):bqac133. doi: 10.1210/endocr/bqac133.PMID: 35957590
- American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update.
Blonde L, Umpierrez GE, Reddy SS, McGill JB, Berga SL, Bush M, Chandrasekaran S, DeFronzo RA, Einhorn D, Galindo RJ, Gardner TW, Garg R, Garvey WT, Hirsch IB, Hurley DL, Izuora K, Kosiborod M, Olson D, Patel SB, Pop-Busui R, Sadhu AR, Samson SL, Stec C, Tamborlane WV Jr, Tuttle KR, Twining C, Vella A, Vellanki P, Weber SL.Endocr Pract. 2022 Oct;28(10):923-1049. doi: 10.1016/j.eprac.2022.08.002. Epub 2022 Aug 11.PMID: 35963508
- Economic outcomes of antiobesity medication use among adults in the United States: A retrospective cohort study.
Watkins S, Toliver JC, Kim N, Whitmire S, Garvey WT.J Manag Care Spec Pharm. 2022 Oct;28(10):1066-1079. doi: 10.18553/jmcp.2022.22116. Epub 2022 Jul 20.PMID: 35856489
- Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial.
Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, Jódar E, Kandler K, Rigas G, Wadden TA, isletWharton S; STEP 5 Study Group.Nat Med. 2022 Oct;28(10):2083-2091. doi: 10.1038/s41591-022-02026-4. Epub 2022 Oct 10.PMID: 36216945
- Extracellular vesicles in β cell biology: Role of lipids in vesicle biogenesis, cargo, and intercellular signaling.
Aguirre RS, Kulkarni A, Becker MW, Lei X, Sarkar S, Ramanadham S, Phelps EA, Nakayasu ES, Sims EK, Mirmira RG.Mol Metab. 2022 Sep;63:101545. doi: 10.1016/j.molmet.2022.101545. Epub 2022 Jul 8.PMID: 35817393
Get to know the UCDC researchers: Saba Alsharif
 In a new series, the UAB Comprehensive Diabetes Center (UCDC), a leader in the field of diabetes research, will highlight its dynamic faculty and trainees.
In a new series, the UAB Comprehensive Diabetes Center (UCDC), a leader in the field of diabetes research, will highlight its dynamic faculty and trainees.
Endocrinology journal selects Shalev Lab research as feature article of the week
 Endocrinology journal selected a recent paper from the research lab of UAB Comprehensive Diabetes Center Director Anath Shalev, M.D., as the feature article of the week. As the feature article, the team’s research will stay at the top of the journal home page for the week.
Endocrinology journal selected a recent paper from the research lab of UAB Comprehensive Diabetes Center Director Anath Shalev, M.D., as the feature article of the week. As the feature article, the team’s research will stay at the top of the journal home page for the week.
Their featured research discovered that deletion of alpha cell TXNIP improves diabetes-associated hyperglucagonemia and glucose homeostasis. The team used alpha cell TXNIP knockout (aTKO) mice and different mouse models of diabetes and glucose intolerance.
Shalev and her lab have found themselves at the forefront of TXNIP research for over two decades. In particular, their lab has been able to demonstrate that the protein TXNIP plays a central role in the disfunction of islet beta cells, which are key to maintaining glucose control due to their production the hormone insulin.
Now, they have found that the role of TXNIP extends beyond the beta cell and also affects other islet cells.
Before it became the Endocrinology feature article, their research “Alpha Cell Thioredoxin-interacting Protein Deletion Improves Diabetes-associated Hyperglycemia and Hyperglucagonemia” was featured in UAB News in September.
Endocrinology is the flagship basic science journal of the Endocrine Society. Journal editors publish research investigating endocrine function at all levels of biological organization.
All study authors include:
Shalev discusses new frontiers in diabetes research with UAB MedCast podcast
 UAB Comprehensive Diabetes Center Director Anath Shalev, M.D., recently sat down with UAB MedCast podcast to talk about new frontiers in diabetes research.
UAB Comprehensive Diabetes Center Director Anath Shalev, M.D., recently sat down with UAB MedCast podcast to talk about new frontiers in diabetes research.
Blum, Toren lead successful dissertation defenses
 One of the UAB Comprehensive Diabetes Center's mission pillars is the training of the next generation of diabetes professionals. In our long-term pursuit of improving the lives of those affected by diabetes, we train undergraduate, graduate students, and postdoctoral scholars as well as clinical fellows in diabetes research and management.
Read more
One of the UAB Comprehensive Diabetes Center's mission pillars is the training of the next generation of diabetes professionals. In our long-term pursuit of improving the lives of those affected by diabetes, we train undergraduate, graduate students, and postdoctoral scholars as well as clinical fellows in diabetes research and management.
Read more
Get to know the UCDC researchers: Sasanka Ramanadham

In a new series, the UAB Comprehensive Diabetes Center (UCDC), a leader in the field of diabetes research, will highlight its dynamic faculty and trainees.
Read moreWhy legacy giving made sense for the Bush family

The year was 1968, and Donnie Bush found himself in a rural doctor’s office facing a Type 1 diabetes diagnosis. This was still a daunting diagnosis at the time: while treatment options were improving, disposable syringes were not yet widely available.
Read moreWhite secures travel award for Bioactive Lipids Conference
 UAB Comprehensive Diabetes Center Graduate Research Assistant and Ph.D. Candidate Tayleur White was awarded a travel award for the 17th Annual Bioactive Lipids in Cancer, Inflammation, and Related Diseases International Conference.
UAB Comprehensive Diabetes Center Graduate Research Assistant and Ph.D. Candidate Tayleur White was awarded a travel award for the 17th Annual Bioactive Lipids in Cancer, Inflammation, and Related Diseases International Conference.
Hunter Lab takes step forward in understanding embryonic pancreatic islet cell development
 Beta-cell regeneration– an area of research that may feel more science fiction than science today. But, it is a keen area of interest for UAB Comprehensive Diabetes Center (UCDC) researchers using cell biology approaches to discover curative treatments for Type 1 and Type 2 diabetes mellitus (T1D, T2D).
Beta-cell regeneration– an area of research that may feel more science fiction than science today. But, it is a keen area of interest for UAB Comprehensive Diabetes Center (UCDC) researchers using cell biology approaches to discover curative treatments for Type 1 and Type 2 diabetes mellitus (T1D, T2D).
Those with diabetes mellitus struggle with chronic high glucose levels, which in T1D, can be attributed to their immune system destroying pancreatic beta-cells. In T2D, these detriments are due to insulin resistance and beta-cell dysfunction. In healthy individuals, beta-cells decrease blood glucose levels through the production and regulation of the hormone, insulin.
There are several approaches to develop novel treatments for diabetes that range from preventive interventions to genetic research approaches, and more. Some researchers attempt to find ways to stop the autoimmune destruction of beta-cells from happening, while others may focus on tempering the alpha-cell, a related islet cell that produces glucagon– a hormone that raises glucose levels.
Division of Endocrinology, Diabetes, and Metabolism Associate Professor and Scientist in the UAB Comprehensive Diabetes Center Chad Hunter, Ph.D., and his lab’s efforts may help inform another strategy to combat diabetes: generation of beta-cells to replace the destroyed or dysfunctional ones.
In their newest publication “The Ldb1 transcriptional co-regulator is required for establishment and maintenance of the pancreatic endocrine lineage,” in the Journal of the Federation of American Societies for Experimental Biology, the authors note the importance of this research: “With the ongoing interest in generating beta and islet-like cells for replacement in diabetic patients, understanding the regulation of embryonic islet cell development remains exceedingly important.”
Authors detail their findings of the role of a protein–Ldb1–as an islet-wide gene regulator of identity and function in the pancreas. Through animal modeling, researchers demonstrated that Ldb1 is necessary for pancreatic islet cell differentiation.
This means that as a cell is generated, this factor helps determine whether a stem cell will become an alpha-cell, beta-cell, or other pancreatic cell type. As a consequence, newborn mice lacking the Ldb1 factor were deficient in beta-cells and therefore insulin, resulting in severe blood glucose impairments.
The significance of the Hunter Lab’s findings is noted in their discussion, “Ldb1 requirement for the emergence of islet cell types during multiple stages of pancreatic development makes it a uniquely valuable tool for our understanding and treatment of diabetes.” These UCDC-investigator-led studies provide new insights into how beta- and islet cells are made.
All study authors, in addition to Hunter, include:
- Eliana Toren, first author, PhD candidate
- Yanping Liu, Ph.D., research scientist
- Maigen Bethea, former Ph.D. student, postdoc fellow at University of Colorado
- Alexa Wade, former UAB undergraduate researcher, Ph.D. candidate at Johns Hopkins
Support came from National Institutes of Health grants DK111483, DK111181 and GM008111; and American Diabetes Association grants 1-16-JDF-044 and 1-17-MUI-004.
The UCDC is a University-Wide Interdisciplinary Research Center comprised of over 200 faculty members from 10 different schools and many departments. It also serves as the umbrella for various research programs and awards; including the prestigious P30 Diabetes Research Center (DRC), U01 Human Islet Research Network (HIRN) grants from the National Institute of Health (NIH) and several research core facilities.
Garvey and team find type 2 diabetes, socioeconomic and social vulnerability disparities predict COVID-19 outcomes
 Department of Nutrition Sciences Professor, Tim Garvey, M.D., and team set out to investigate the effects of both cardiometabolic disease (CMD)–such as obesity and type 2 diabetes–and social determinants of health (SDoH)–like educational attainment, social vulnerability index, rurality, and healthcare access– on COVID-19 outcomes.
Department of Nutrition Sciences Professor, Tim Garvey, M.D., and team set out to investigate the effects of both cardiometabolic disease (CMD)–such as obesity and type 2 diabetes–and social determinants of health (SDoH)–like educational attainment, social vulnerability index, rurality, and healthcare access– on COVID-19 outcomes.
Researchers discover rise in new-onset type 2 diabetes among Alabama youth during COVID-19 pandemic
 Researchers at the University of Alabama at Birmingham have recently discovered a rise in new-onset type 2 diabetes among Alabama youth during COVID-19 pandemic. Particularly, they found that the increase in cases disproportionately affected Medicaid enrollees and males.
Researchers at the University of Alabama at Birmingham have recently discovered a rise in new-onset type 2 diabetes among Alabama youth during COVID-19 pandemic. Particularly, they found that the increase in cases disproportionately affected Medicaid enrollees and males.
UCDC excited to work with UAB Heersink School of Medicine on major strategic recruitment initiative in diabetes research
 As cool mornings begin to break up the summer heat and fall quickly approaches, the UAB Comprehensive Diabetes Center (UCDC) is partnering with the UAB Heersink School of Medicine and campus departments to identify and recruit top talent in the field of diabetes research.
As cool mornings begin to break up the summer heat and fall quickly approaches, the UAB Comprehensive Diabetes Center (UCDC) is partnering with the UAB Heersink School of Medicine and campus departments to identify and recruit top talent in the field of diabetes research.
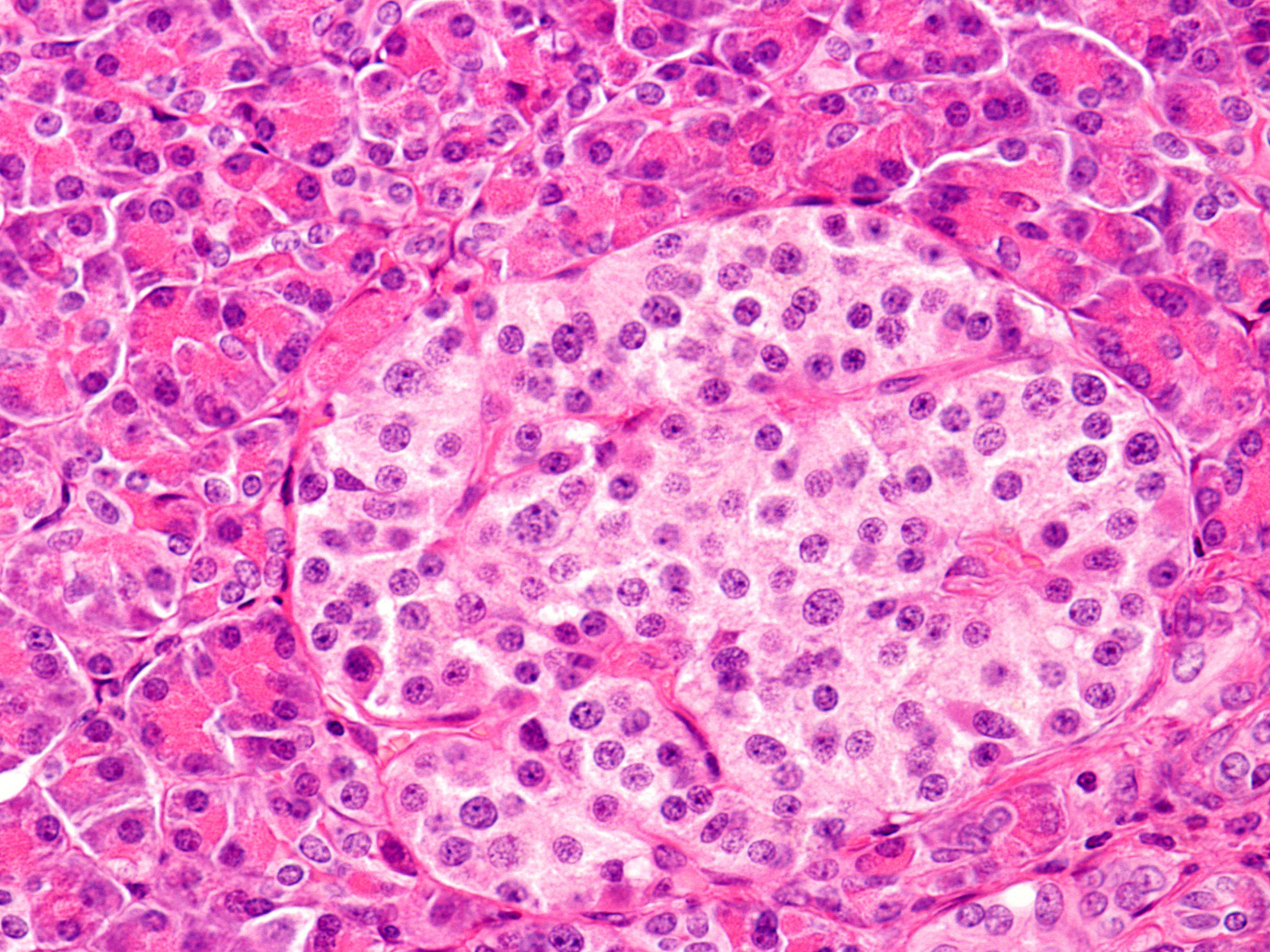
Looking beyond the beta cell: UCDC lab investigates effects of alpha cell TXNIP deletion
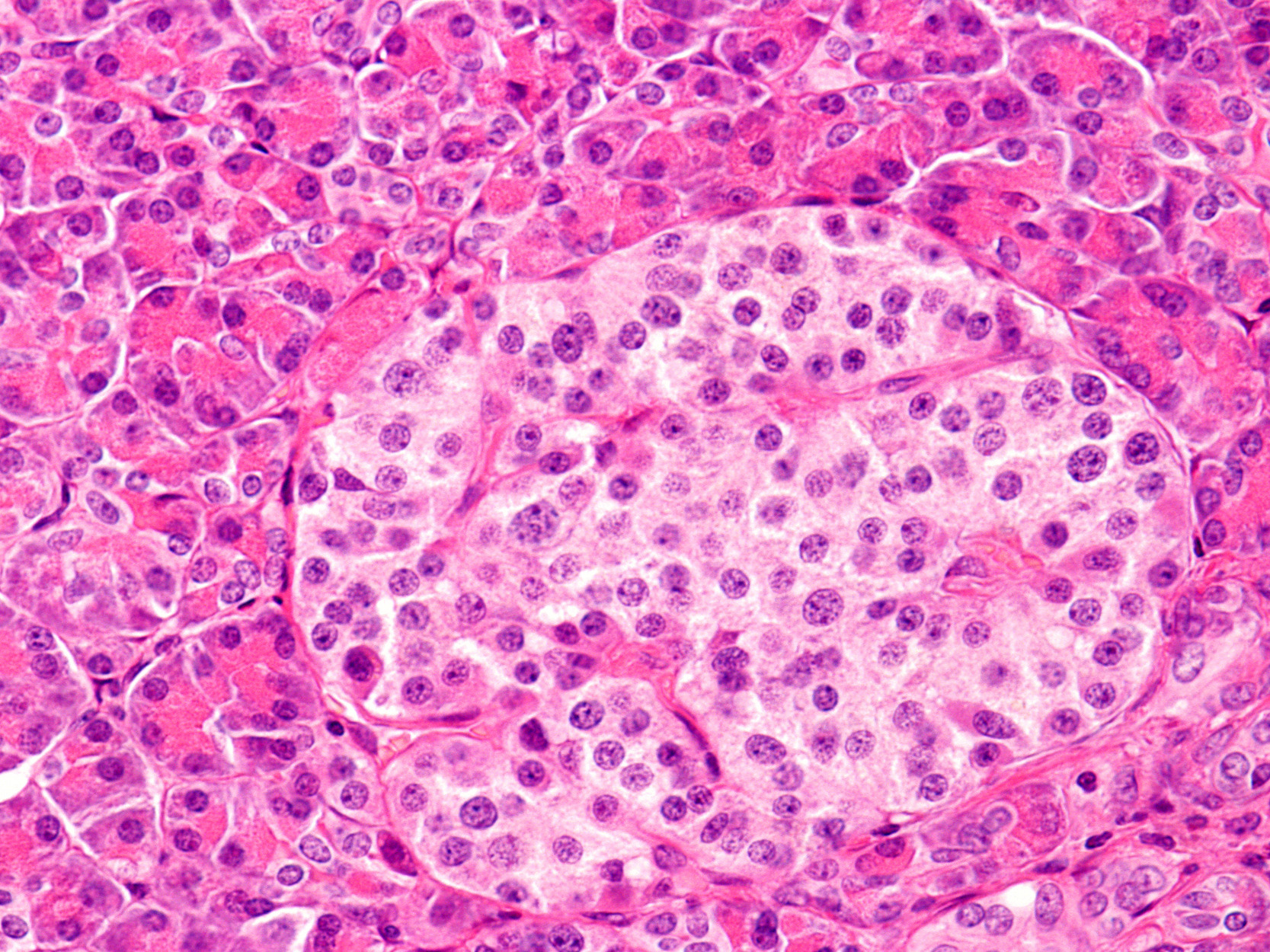 Many researchers are pursing treatment options for diabetes—a disease characterized by high glucose levels— through approaches that increase insulin. However, there are two pancreatic hormones that play a critical role in regulating glucose levels, insulin, produced by pancreatic beta cells, lowers glucose and glucagon, produced by pancreatic alpha cells, increases circulating glucose levels.
Many researchers are pursing treatment options for diabetes—a disease characterized by high glucose levels— through approaches that increase insulin. However, there are two pancreatic hormones that play a critical role in regulating glucose levels, insulin, produced by pancreatic beta cells, lowers glucose and glucagon, produced by pancreatic alpha cells, increases circulating glucose levels.
Get to know the UCDC researchers: Eliana Toren
 In a new series, the UAB Comprehensive Diabetes Center (UCDC), a leader in the field of diabetes research, will highlight its dynamic faculty and trainees.
In a new series, the UAB Comprehensive Diabetes Center (UCDC), a leader in the field of diabetes research, will highlight its dynamic faculty and trainees.
The UCDC has members from across several schools and departments at UAB. Diabetes research capabilities are made stronger by the expansive research focuses and innovations that members bring to the table. To better understand these research capabilities and discoveries, we will spotlight UCDC researchers throughout the year.
Read moreUCDC is proud to support endocrinology and diabetes national ranking
 U.S. News & World Report (USNWR) recently ranked UAB Hospital as the best hospital in Alabama, naming several of its specialties as top-ranking in the nation as well. Of the rankings, UAB Hospital’s endocrinology and diabetes specialty came in a number 40 in the nation.
U.S. News & World Report (USNWR) recently ranked UAB Hospital as the best hospital in Alabama, naming several of its specialties as top-ranking in the nation as well. Of the rankings, UAB Hospital’s endocrinology and diabetes specialty came in a number 40 in the nation.
UCDC finds a novel factor involved in diabetic beta cell stress
 Dr. Guanlan Xu, corresponding author. Behold the beta-cell: a cell found in the islets of Langerhans in the pancreas that is responsible for all the insulin production and secretion needed to maintain normal blood sugar levels.
Dr. Guanlan Xu, corresponding author. Behold the beta-cell: a cell found in the islets of Langerhans in the pancreas that is responsible for all the insulin production and secretion needed to maintain normal blood sugar levels.